Rapier
- 83
- 0
Homework Statement
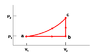
An ideal monatomic gas is taken through the above cycle where p1= 2 X 105 Pa and V1 = 5 cm3. During the cycle both the pressure and volume will change such that p2 = 2 p1 and V2 = 4 V1.
a) How much work is done by the gas in going from state a to state c along path abc?
Wabc = 3 J
OK
b) What is the change in internal energy in going from b to c?
Ubc= J
c) What is the change in internal energy during the complete cycle?
UTOT = 0 J
OK
d) What is the change in entropy per mole of gas in going from b to c?
ΔSbc = J/K
e) What is the change in entropy per mole during the complete cycle?
ΔSTOT= 0 J/K
OK
I am having problems with Parts B and D.
Homework Equations
PV=NRT
Q = nCvdt
The Attempt at a Solution
I only have P and V. I cannot use the Ideal Gas Law to calculate n or T because I have two unknowns. I cannot use my Heat Equations because I am still missing n and T. I'm just stuck. If I can calculate B, I think I can get D...I just don't see any way to calculate the Heat in part B. HELP!
Thanks.