zag4life
- 2
- 0
When a system is taken from state i to state f along path iaf in the figure, Q = 60 cal and W = 10 cal. Along path ibf, Q = 65 cal.
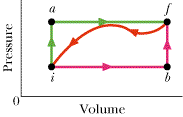
(a) What is W along path ibf? (b) If W = -19 cal for the return path fi, what is Q for this path? (c) If Eint,i = 11 cal, what is Eint,f?(d) If Eint,b = 27 cal what is Q for path ib?(e) For the same value of Eint,b, what is Q for path bf?
Eint= internal energy of the system; Q= heat; W= work
I have used Eint= Q - W to solve most of the problem.
I only am marked wrong on part d which is very frustrating. So far I have:
(a)Wibf = 15 cal because W= Q-E (b) Qfi= -69 cal because Q= -(E)+W (c) Eint,f= 61 cal because Eint,f= Eint+Ei (d) Qib= ? (e) Qbf= 34 because Eint,f =E-Eint,b
Any thoughts?
(a) What is W along path ibf? (b) If W = -19 cal for the return path fi, what is Q for this path? (c) If Eint,i = 11 cal, what is Eint,f?(d) If Eint,b = 27 cal what is Q for path ib?(e) For the same value of Eint,b, what is Q for path bf?
Homework Equations
Eint= internal energy of the system; Q= heat; W= work
I have used Eint= Q - W to solve most of the problem.
The Attempt at a Solution
I only am marked wrong on part d which is very frustrating. So far I have:
(a)Wibf = 15 cal because W= Q-E (b) Qfi= -69 cal because Q= -(E)+W (c) Eint,f= 61 cal because Eint,f= Eint+Ei (d) Qib= ? (e) Qbf= 34 because Eint,f =E-Eint,b
Any thoughts?
Last edited: