- #1
mech-eng
- 828
- 13
When a gas in a closed system is compressed, should the graph always be non-linear? If T is constant ie if the process isothermal it is clear that it should be so but I am not very sure that if the graph can be horizontal. If T decreases might not it be horizontal?
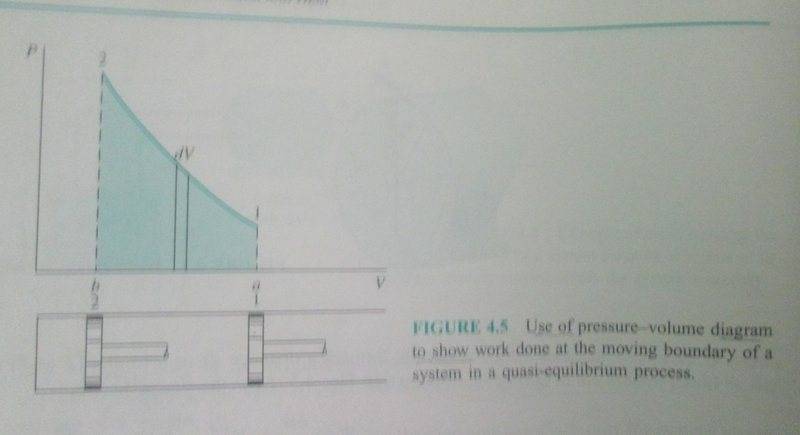
Thank you.
Thank you.