Epictetus
- 5
- 0
Homework Statement
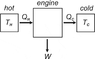
Suppose that two heat engines are connected in a series, such that the heat exhaust of the first engine is used as the heat input of the second (attached diagram below). The efficiencies of the engines are e1 and e2, respectively. Show that the net efficiency of the combination is given by:
e(net)= e1 + (1-e1)e2
Homework Equations
e(max)= 1 - Tc/Th
e= w/Qh = Qh-Qc/Qh = 1 - Qc/Qh
Qc/Qh = Tc/Th
The Attempt at a Solution
I broke up the diagram into two free-body diagrams allowing me to for solve e1 and e2:
e1 = 1-Th/Tm (Am I allowed to apply Qc/Qh = Tc/Th into 1 - Qc/Qh ?)
and
e2= 1-Tm/Tc
Applying e1 and e2 in the given equation:
e(net)= e1 + (1-e1)e2
= (1-Th/Tm) + [1-(1-Th/Tm)](1-Tm/Tc)
which leaves
=1-Tm/Tc (The total efficiency of the engine equals to only the second engine because all the heat input eventually ends up there? )