You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Help with finding chirality centres
- Thread starter andrewvidler
- Start date
-
- Tags
- Chirality
AI Thread Summary
Identifying chiral centers in complex molecules can be challenging, especially when multiple chirality centers are present. A carbon atom is considered chiral if it has four different substituents. To determine chirality, one should evaluate the substituents by moving outward from the chiral carbon and comparing the atoms encountered. If the paths to two substituents converge or show no differences, the carbon is not chiral. Understanding how far to trace along branches to identify different substituents is crucial for accurate chirality assessment.
Physics news on Phys.org
Borek
Mentor
- 29,123
- 4,541
What methods of recognizing chiral carbons do you know?
Please note that attaching .doc files is not a good idea - many people are reluctant to open them, as they can contain viruses. In such a case best approach is to attach an image.
Edit: I think I am bored, I have replaced doc with png in your post.
Please note that attaching .doc files is not a good idea - many people are reluctant to open them, as they can contain viruses. In such a case best approach is to attach an image.
Edit: I think I am bored, I have replaced doc with png in your post.
andrewvidler
- 5
- 0
Borek
Mentor
- 29,123
- 4,541
Perhaps you are thrown off by cycles?
Select an atom, move out from it in each direction and compare atoms on the way. If you get to the same place from both sides and there were no differences on the way - atom is not chiral.
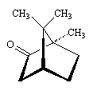
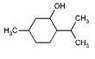
Try it on some simple molecules first, say cyclohexanol and 2-methylcyclohexanol.
Select an atom, move out from it in each direction and compare atoms on the way. If you get to the same place from both sides and there were no differences on the way - atom is not chiral.
Try it on some simple molecules first, say cyclohexanol and 2-methylcyclohexanol.
andrewvidler
- 5
- 0
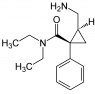
Hi - how about this one? I am confused as to what constitutes a different group. I mean how far do go along the separate branches from the ceontre to say that is a differernt substituent.
this is one where it days there are 3. I know where they are but i don't see why
this is one where it days there are 3. I know where they are but i don't see why
Attachments
Borek
Mentor
- 29,123
- 4,541
andrewvidler said:how far do go along the separate branches from the centre to say that is a differernt substituent
Either till you "meet" or till you find a difference - whichever comes first.
TL;DR Summary: cannot find out error in solution proposed.
[![question with rate laws][1]][1]
Now the rate law for the reaction (i.e reaction rate) can be written as:
$$ R= k[N_2O_5] $$
my main question is, WHAT is this reaction equal to?
what I mean here is, whether
$$k[N_2O_5]= -d[N_2O_5]/dt$$
or is it
$$k[N_2O_5]= -1/2 \frac{d}{dt} [N_2O_5] $$ ?
The latter seems to be more apt, as the reaction rate must be -1/2 (disappearance rate of N2O5), which adheres to the stoichiometry of the...
I don't get how to argue it. i can prove: evolution is the ability to adapt, whether it's progression or regression from some point of view, so if evolution is not constant then animal generations couldn`t stay alive for a big amount of time because when climate is changing this generations die. but they dont. so evolution is constant.
but its not an argument, right?
how to fing arguments when i only prove it.. analytically, i guess it called that (this is indirectly related to biology, im...
Similar threads
- Replies
- 1
- Views
- 2K
- Replies
- 1
- Views
- 1K
- Replies
- 1
- Views
- 3K
- Replies
- 15
- Views
- 3K
- Replies
- 1
- Views
- 2K
- Replies
- 4
- Views
- 3K
- Replies
- 1
- Views
- 2K
- Replies
- 3
- Views
- 15K
- Replies
- 2
- Views
- 2K
- Replies
- 13
- Views
- 2K
Hot Threads
-
Confusion regarding a chemical kinetics problem
- Started by palaphys
- Replies: 12
- Biology and Chemistry Homework Help
-
Biology Prove that evolution is constant (Provide at least two arguments to support your position)
- Started by yo yo
- Replies: 24
- Biology and Chemistry Homework Help
Recent Insights
-
Insights Quantum Entanglement is a Kinematic Fact, not a Dynamical Effect
- Started by Greg Bernhardt
- Replies: 7
- Quantum Physics
-
Insights What Exactly is Dirac’s Delta Function? - Insight
- Started by Greg Bernhardt
- Replies: 0
- General Math
-
Insights Relativator (Circular Slide-Rule): Simulated with Desmos - Insight
- Started by Greg Bernhardt
- Replies: 1
- Special and General Relativity
-
Insights Fixing Things Which Can Go Wrong With Complex Numbers
- Started by PAllen
- Replies: 7
- General Math
-
Insights Fermat's Last Theorem
- Started by fresh_42
- Replies: 91
- General Math
-
Insights Why Vector Spaces Explain The World: A Historical Perspective
- Started by fresh_42
- Replies: 0
- General Math