When thinking about the conformational changes of proteins, biophysicists like to talk about the concept of an
energy landscape. As you may have leaned in chemistry class when discussing thermodynamics, systems tend to want to find a state of minimum free energy, and the difference in free energy between different states can tell you about the relative amounts of the different states at equilibrium (i.e. the equilibrium constant). Chemists often visualize the energy landscape of a chemical chemical reactions as something like the picture below:
Where the reactants are associated with a certain free energy, the products are associated with a lower free energy (with the difference being the ΔG of the reaction), and the two are separated by an energy barrier (E
a, or the activation energy). The x-axis of the plot represents the "reaction coordinate" and in this case it represents the whether the intermediate is more reactant-like or product-like.
Biophysicists view protein folding from a the similar view of an energy landscape, but instead of a one-dimensional reaction coordinate, protein folding occurs in a very highly multidimensional space (one can think of a polypeptide as a flexible chain where each amino acid has two "joints" around which the chain can rotate to achieve the different conformations). Here, each point of the energy landscape represents the thermodynamic stability (i.e. the free energy) of a particular protein conformation. Here is one artists visualizaiton of the energy landscape of protein folding (sometimes referred to as a "folding funnel"). High energy structures (i.e. unstable) are at the top, and the low energy conformations are at the bottom:
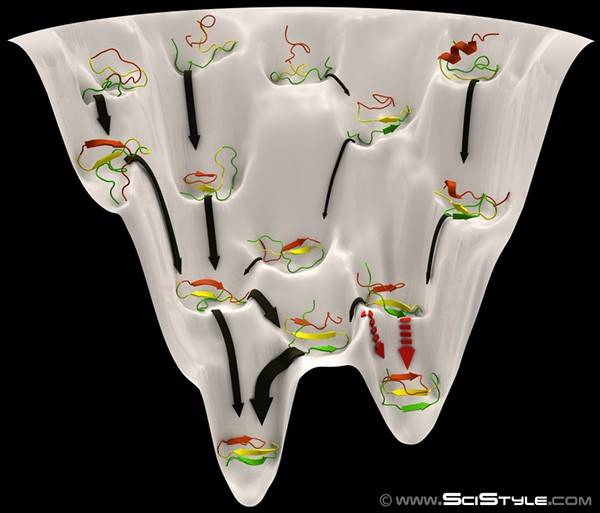
Similarly, conformational changes can then be thought of as the meandering of protein around on the energy landscape. Because the energy landscape depends on the interaction energies between all of the atoms in the protein, changing the environment, for example, by adding a ligand that interacts with the protein, can change the energy landscape and affect both the most stable conformation at equilibrium as well as the "excited" states that are accessible:
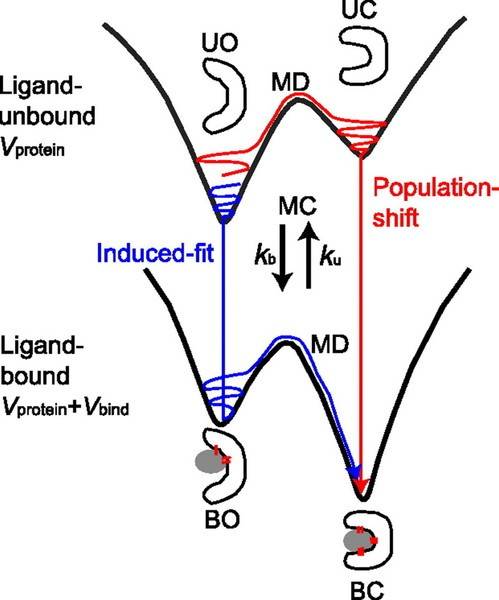
For example, the image above shows a protein that can exist in two different conformations: an open conformation (O) and a closed conformation (C). In the absence of ligand when the protein is unbound (U), the open state has lower energy, so the protein mostly stays open. However, the barrier between the two states is low enough that thermal energy can sometimes excite the protein into the higher energy closed state. However, the presence of the ligand changes the thermodynamics such that in the bound state (B), the closed conformation now has the lower energy, and the open conformation is the excited state.
If you are interested more in the topic, the Molecular Driving Forces textbook by Ken Dill is a good reference to learn the basics.