- #1
TFM
- 1,026
- 0
Homework Statement
In the derivation of the energy levels in the hydrogen atom one commonly assumes that the nucleus is a point charge. However, in reality the size of the nucleus is of the order of [tex] 1fm=10^−15m[/tex]. Since this is very much smaller than the typical distance of the electron from the nucleus, which is of the order of [tex] a_0 \approx 0.5 Angstoms = 0.5 *10^{-10}m [/tex], the finite size of the nucleus can be taken into account perturbatively.
a)
Assume that the nucleus of the hydrogen atom is a uniformly charged spherical shell of radius [tex] \delta [/tex]. According to Gauss’ law [tex] \int E \cdot dA = Q_{enclosed}/\epsilon_0[/tex], the electric field outside this shell is the same as for a point charge at the centre. However, from Gauss’ law also follows that the electric field
inside the shell is zero. Since the force [tex]-eE_r[/tex] on the electron is given by the gradient [tex] -\partial V/\partial r [/tex] of the potential, the potential must thus be constant inside the shell and indistinguishable from the Coulomb potential outside it.
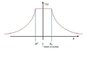
Sketch the potential V (r), find the expression for it, and then find the perturbation [tex] \Delta [/tex]V relative to the Coulomb potential generated by a point-like nucleus.
b)
Use the ground-state wave function for a hydrogen atom with a point-like nucleus,
[tex] \Phi_{100}(r,\theta, \phi) = \frac{1}{\sqrt{\pi a_0^3}}e^{-r/a_0} [/tex]
and calculate the shift of the ground-state energy due to the distribution of the nuclear charge overa finite spherical shell to first order in the perturbation.
c)
Expand the result of (b) in powers of [tex] \delta/a_0 [/tex], retain only the first non-vanishing term, and thus show that the ground-state energy shift is approximately
[tex] \Delta E_1 \approx \frac{e^2}{6\pi\epsilon_0}\frac{\delta^2}{a_0^3} [/tex]
Homework Equations
Given on sheet:
[tex] e^x = \Sigma \frac{x^n}{n!}[/tex]
The Attempt at a Solution
Okay B, I think is a normal inverse square law graph, similar to that of gravity. It goes up with constant gradient until it reaches the radius R, then decreases inverse square law curve.
I am not sure however what to do for b). Do I need to insert the potential into a Schrödinger Equation?