That description appears to be quoted from
https://sciencing.com/happens-salt-added-water-5208174.html,
which says what you quoted:
but also immediately following that says:That source cites this one as a reference ##-##
https://www.usgs.gov/media/images/water-molecules-and-their-interaction-salt-molecules:
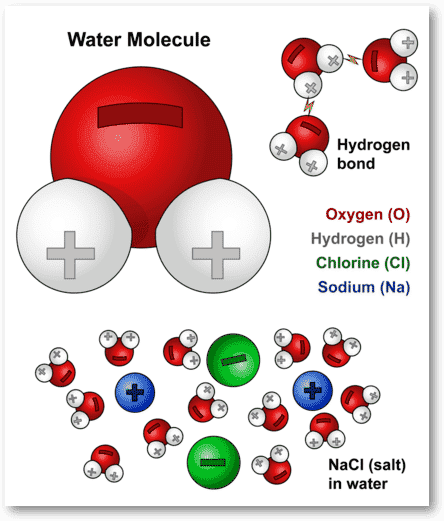
Water molecules and their interaction with salt molecules
This diagram shows the positive and negative parts of a water molecule. It also depicts how a charge, such as on an ion (Na or Cl, for example) can interact with a water molecule.
At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges on opposite sides in the molecule. The bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively charged and the sodium ion is positively charged. Likewise, a water molecule is ionic in nature, but the bond is called covalent, with two hydrogen atoms both situating themselves with their positive charge on one side of the oxygen atom, which has a negative charge. When salt is mixed with water, the salt dissolves because the covalent bonds of water are stronger than the ionic bonds in the salt molecules.
The positively-charged side of the water molecules are attracted to the negatively-charged chloride ions and the negatively-charged side of the water molecules are attracted to the positively-charged sodium ions. Essentially, a tug-of-war ensues with the water molecules winning the match. Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
Although both sources say that it is the sodium and chloride ions, and not the components of the water molecule, that are pulled apart (which is something that
@russ_watters succinctly pointed out), there is an important difference in the description of the ionic interactions ##-## repeating from the sciencing.com source:
The second sentence is incorrect (due to a probably-inadvertent repetition of "electronegative oxygen portion" from the preceding sentence) ##-## it would not have been incorrect to say that the negatively charged chlorine portion of NaCl is attracted to the positively charged hydrogen portions of the water molecule, which is probably what the writer intended.