- #1
beibixx
- 8
- 0
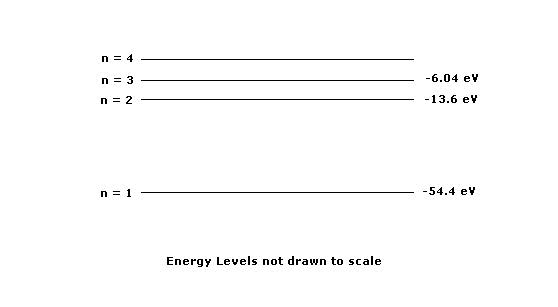
1. The diagram above shows the lowest four descrete energy levels of an atom. An electron in the n=4 state makes a transition to the n=2 state, emitting a photon of wavelength 121.9 nm
(a)Calculate the energy level of the n=4 state.
(b)Calbulate the momentum of the photon.
The photon is then incident on a silver surface in a photoelectric experiment, and the surface emits an electron with maximum possible kinetic energy. The work function of the silver is 4.7eV
(c) Calculate the kinetic energy, in eV, of the emitted electron.
 2. E=hf
2. E=hf
E= pc
h=6.63 x 10[tex]^-34[/tex]J.s
h=4.14 x 10[tex]^-15[/tex]eVs
KEmax = hf-work function
work function = hf0
v=fλ
λ=h/p3. ...might come back with this..
(a)Calculate the energy level of the n=4 state.
(b)Calbulate the momentum of the photon.
The photon is then incident on a silver surface in a photoelectric experiment, and the surface emits an electron with maximum possible kinetic energy. The work function of the silver is 4.7eV
(c) Calculate the kinetic energy, in eV, of the emitted electron.
E= pc
h=6.63 x 10[tex]^-34[/tex]J.s
h=4.14 x 10[tex]^-15[/tex]eVs
KEmax = hf-work function
work function = hf0
v=fλ
λ=h/p3. ...might come back with this..
Last edited: