- #1
sues
- 4
- 0
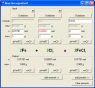
1.0 g of Iron metal reacts with 2.0 g of chlorine gas to make iron (III) chloride, FeCl3.
(a) Write a balanced equation for this reaction.
(b) Which of the reactants is present in excess ?
(c) What mass of this reactant will be left over at the end of the reaction ? (7 marks)
OK with equation : 2Fe + 3Cl2 = 2FeCl3
Went for moles of Fe as 2/112 and moles of Cl2 as 2/213 then didn't know what to do with numbers
(a) Write a balanced equation for this reaction.
(b) Which of the reactants is present in excess ?
(c) What mass of this reactant will be left over at the end of the reaction ? (7 marks)
Homework Equations
OK with equation : 2Fe + 3Cl2 = 2FeCl3
The Attempt at a Solution
Went for moles of Fe as 2/112 and moles of Cl2 as 2/213 then didn't know what to do with numbers