DivGradCurl
- 364
- 0
Hi all,
I came across this figure in a textbook. Simple stuff, but can get tricky:
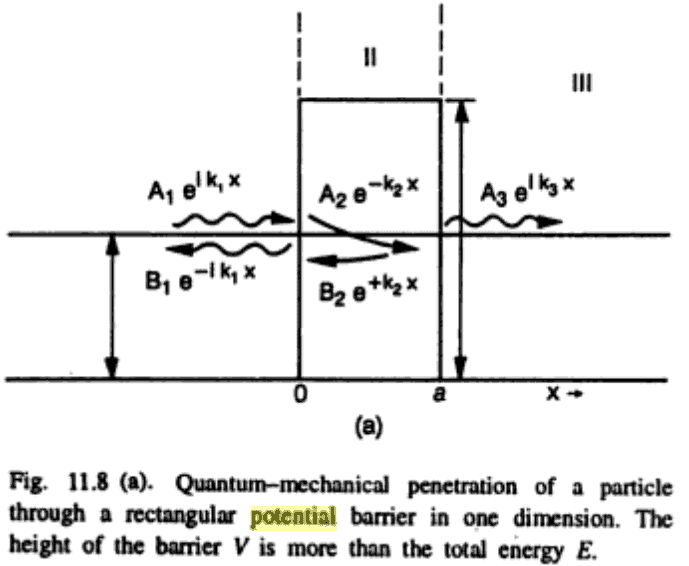
I don't understand why the sign convention flips upon entering the barrier (region II), but I guess the book is correct and that I should just take it as a fact.
If anybody has a reasonable thought to add so I'm making sense out of this rather than just accepting it, please let me know.
Thanks!
I came across this figure in a textbook. Simple stuff, but can get tricky:
I don't understand why the sign convention flips upon entering the barrier (region II), but I guess the book is correct and that I should just take it as a fact.
If anybody has a reasonable thought to add so I'm making sense out of this rather than just accepting it, please let me know.
Thanks!