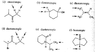- #1
Helicore
- 1
- 0
I am a bit confused with how to determine whether a set of hydrogens are enantiomers or diastereomers (and therefore how many different sets there are).
[In attachment} I understand d, and e, since they both already have a chirality center, and replacing a hydrogen on the CH2 (which is a pro-chirality center) would create another chirality center making it a diastereomer. But I don't quite get the rest when there are no present chirality centers.
I am assuming a is enantiotopic since it would produce a single chirality center (?), but the other two diastereotopic ones don't quite make sense to me (though I think it should..).
For the CH3's that make up the diastereomer in c, and e, the hydrogens on the methyl itself are homotopic correct? And diastereotopic when compared to the other methyl?
* What kind of process should I be going through to determine this?
I think I have only a single step so far that I am somewhat sure on, which is to check for any present chirality centers. What should follow?
[In attachment} I understand d, and e, since they both already have a chirality center, and replacing a hydrogen on the CH2 (which is a pro-chirality center) would create another chirality center making it a diastereomer. But I don't quite get the rest when there are no present chirality centers.
I am assuming a is enantiotopic since it would produce a single chirality center (?), but the other two diastereotopic ones don't quite make sense to me (though I think it should..).
For the CH3's that make up the diastereomer in c, and e, the hydrogens on the methyl itself are homotopic correct? And diastereotopic when compared to the other methyl?
* What kind of process should I be going through to determine this?
I think I have only a single step so far that I am somewhat sure on, which is to check for any present chirality centers. What should follow?