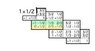
- #1
bdizzle329
- 2
- 0
An electron in a hydrogen atom occupies the combined position and spin state.
[tex]\Psi[/tex][tex]\left(\vec{r},\xi\right)[/tex]=[tex]\left(\sqrt{1/3}Y^{1}_{0}\xi_{+}+\sqrt{2/3}Y^{1}_{1}\xi_{-}\right)[/tex]
What are the possible measured values of [tex]J^{2}[/tex] (where J is the total angular momentum of the electron L + S) and with what probability will each be found?
[tex]J^{2}[/tex] = [tex]\hbar^{2}j\left(j+1\right)[/tex]
[tex]\left|l-s\right|\geq j \geq l+s [/tex] , where [tex]l[/tex] and [tex]s[/tex] are the orbital angular momentum and spin angular momentum quantum numbers, respectively.
I know that, according to the given position state of the electron and the fact that it is an electron, [tex]l = 1[/tex] and [tex]s = 1/2[/tex].
I know that [tex]j[/tex] will be [tex]1/2[/tex] or [tex]3/2[/tex]. Therefore, [tex]J^{2}[/tex], when measured, will be either [tex]3/4\hbar^{2}[/tex] or [tex]15/4\hbar^{2}[/tex].
I am having trouble determining the associated probabilities of the possible measurement values for [tex]J^{2}[/tex]. From what I have read about addition of angular momentum, it would seem that I would need to calculate the Clebsch-Gordon coefficients for the total angular momentum. I am not really sure where to start. I have read Griffiths' explanation for angular momentum and the Clebsch-Gordon coefficients but he doesn't explain how to use them for total angular momentum.
I feel dumb asking this kind of question but I am having trouble understanding Quantum Mechanics.
[tex]\Psi[/tex][tex]\left(\vec{r},\xi\right)[/tex]=[tex]\left(\sqrt{1/3}Y^{1}_{0}\xi_{+}+\sqrt{2/3}Y^{1}_{1}\xi_{-}\right)[/tex]
What are the possible measured values of [tex]J^{2}[/tex] (where J is the total angular momentum of the electron L + S) and with what probability will each be found?
[tex]J^{2}[/tex] = [tex]\hbar^{2}j\left(j+1\right)[/tex]
[tex]\left|l-s\right|\geq j \geq l+s [/tex] , where [tex]l[/tex] and [tex]s[/tex] are the orbital angular momentum and spin angular momentum quantum numbers, respectively.
I know that, according to the given position state of the electron and the fact that it is an electron, [tex]l = 1[/tex] and [tex]s = 1/2[/tex].
I know that [tex]j[/tex] will be [tex]1/2[/tex] or [tex]3/2[/tex]. Therefore, [tex]J^{2}[/tex], when measured, will be either [tex]3/4\hbar^{2}[/tex] or [tex]15/4\hbar^{2}[/tex].
I am having trouble determining the associated probabilities of the possible measurement values for [tex]J^{2}[/tex]. From what I have read about addition of angular momentum, it would seem that I would need to calculate the Clebsch-Gordon coefficients for the total angular momentum. I am not really sure where to start. I have read Griffiths' explanation for angular momentum and the Clebsch-Gordon coefficients but he doesn't explain how to use them for total angular momentum.
I feel dumb asking this kind of question but I am having trouble understanding Quantum Mechanics.