Coolster7
- 14
- 0
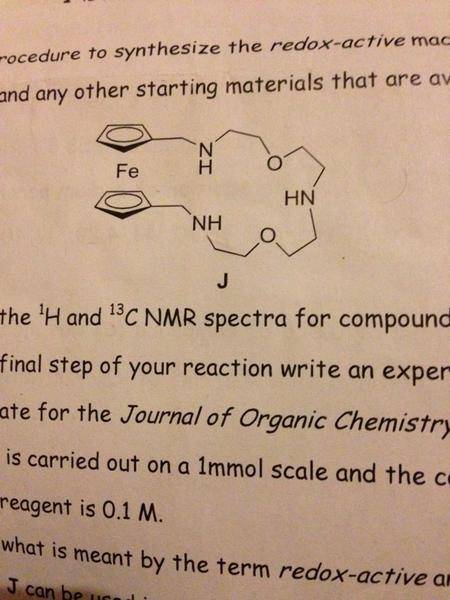
I've been asked to synthesise this ferrocene based macrocycle (shown in picture below) starting from ferrocene and other available materials from Aldrich. I need to make sure molar equivalents are spot on with reagents. Can anyone help please?