- #1
WWCY
- 479
- 12
Hi all, I have an issue trying to understand the following paragraph from Blundell's book.
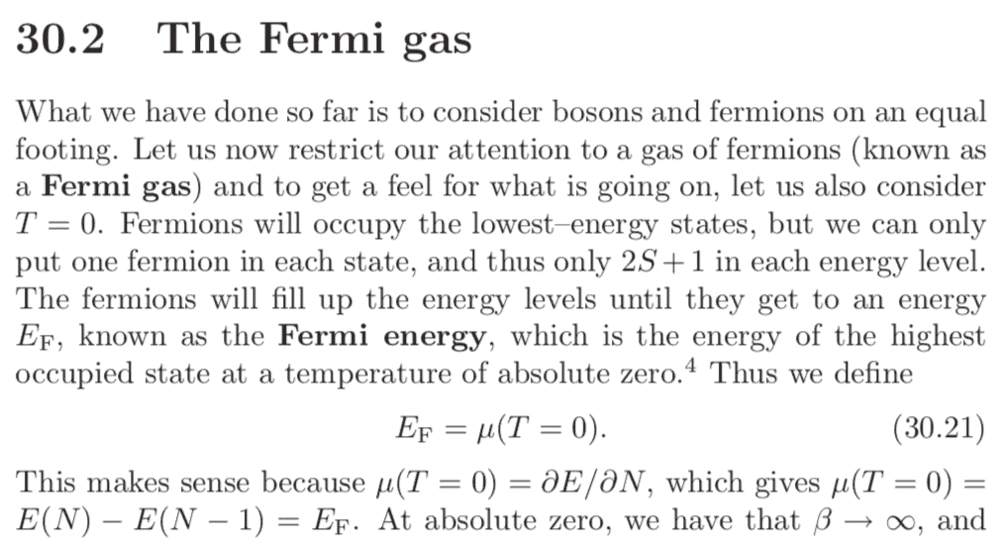
How, exactly, does the definition of ##\mu_0 = E_F## "make sense"? In the sentence after 30.21, it seems to say that the mean energy for a system with ##N## particles differs from that of a system with ##N-1## particles by the highest occupy-able energy level at ##T = 0##, which is ##E_f##. What does this mean?
Many thanks in advance.
How, exactly, does the definition of ##\mu_0 = E_F## "make sense"? In the sentence after 30.21, it seems to say that the mean energy for a system with ##N## particles differs from that of a system with ##N-1## particles by the highest occupy-able energy level at ##T = 0##, which is ##E_f##. What does this mean?
Many thanks in advance.
