- #1
WWCY
- 479
- 12
Hi all, I have been having some issues trying to show that a reversible expansion of gas does not create new entropy. Assistance is greatly appreciated!
So suppose that a gas expands reversibly as shown below at fixed temperature
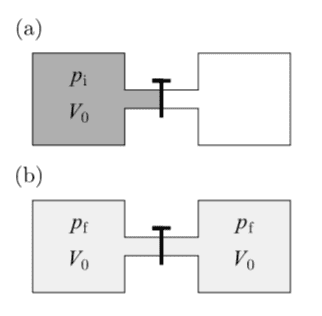
At fixed temperature, internal energy doesn't change so ##dU_{sys} = 0##, which means
$$dS_{sys} = \frac{pdV}{T}$$
swapping ##p/T## using the ideal gas law and integrating from ##V_0## to ##2V_0## gives the change in entropy of the system
$$\Delta S_{sys} = nR\ln{2}$$
In my text (Blundell), the authors say that because this reaction has been defined to be reversible, the change in the entropy of the surroundings must be ##\Delta S_{su} = \Delta S_{sys}##. I have been trying to show this and have been unsuccessful. What I tried was the following:
Conservation of energy means that ##dU_{sys} = -dU_{su} = 0##. The thermodynamic equation for the surrounding is then,
$$T_{su} dS_{su} = p_{su}dV_{su}$$
I supposed, then ##T_{su}## should be equal to ##T_{sys}##, since it was not specified that the container was thermally isolated. This left me with
$$dS_{su} = \frac{1}{T}p_{su}dV_{su} = nR\frac{1}{V_{su}}dV_{su}$$
Then it struck me that I had no clue what the "surrounding" actually was. If it refers to the space outside the container, ##dV_{surr} = 0## which means no change in surrounding entropy, which is clearly wrong. If i consider it to be the evacuated space that the gas expands into...it doesn't look right as well.
I know that ##dS_{total} = 0##, but due to the fact that identifying what's reversible and what's not is a tricky business for me, I would prefer to work things out explicitly to see if total entropy changes
Could someone point out where I went wrong and how I should have worked this out?
Many thanks!
So suppose that a gas expands reversibly as shown below at fixed temperature
At fixed temperature, internal energy doesn't change so ##dU_{sys} = 0##, which means
$$dS_{sys} = \frac{pdV}{T}$$
swapping ##p/T## using the ideal gas law and integrating from ##V_0## to ##2V_0## gives the change in entropy of the system
$$\Delta S_{sys} = nR\ln{2}$$
In my text (Blundell), the authors say that because this reaction has been defined to be reversible, the change in the entropy of the surroundings must be ##\Delta S_{su} = \Delta S_{sys}##. I have been trying to show this and have been unsuccessful. What I tried was the following:
Conservation of energy means that ##dU_{sys} = -dU_{su} = 0##. The thermodynamic equation for the surrounding is then,
$$T_{su} dS_{su} = p_{su}dV_{su}$$
I supposed, then ##T_{su}## should be equal to ##T_{sys}##, since it was not specified that the container was thermally isolated. This left me with
$$dS_{su} = \frac{1}{T}p_{su}dV_{su} = nR\frac{1}{V_{su}}dV_{su}$$
Then it struck me that I had no clue what the "surrounding" actually was. If it refers to the space outside the container, ##dV_{surr} = 0## which means no change in surrounding entropy, which is clearly wrong. If i consider it to be the evacuated space that the gas expands into...it doesn't look right as well.
I know that ##dS_{total} = 0##, but due to the fact that identifying what's reversible and what's not is a tricky business for me, I would prefer to work things out explicitly to see if total entropy changes
Could someone point out where I went wrong and how I should have worked this out?
Many thanks!