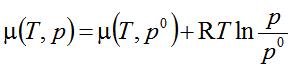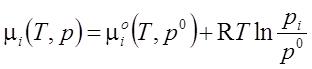Discussion Overview
The discussion revolves around the concept of chemical potential in the context of ideal gas mixtures, specifically addressing the relationship between partial pressure and chemical potential in a perfect mixture of ideal gases.
Discussion Character
- Technical explanation
- Conceptual clarification
- Debate/contested
Main Points Raised
- One participant seeks clarification on the inclusion of variables pi, μi, and μiο in the equation related to chemical potential.
- Another participant defines a perfect mixture, stating that the partial pressure of a component equals the pressure of the pure component in equilibrium contact with the mixture through a semipermeable membrane.
- A participant requests guidance on how to prove the equation, indicating a desire for a starting point in the derivation.
- It is noted that if the gas in the mixture and the free gas are in equilibrium with equal total pressures, then their chemical potentials must also be equal and depend similarly on their respective pressures.
- A reference is provided to a specific chapter in a textbook on chemical engineering thermodynamics for further reading.
Areas of Agreement / Disagreement
Participants express varying levels of understanding and seek clarification, indicating that the discussion remains unresolved with multiple viewpoints on the proof and implications of the equation.
Contextual Notes
Participants have not reached a consensus on the proof of the equation, and there are assumptions regarding equilibrium conditions that have not been fully explored.