nobahar
- 482
- 2
Hello!
I'm not sure if this should be in the physics section or chemistry, but I think it's covered in both. I apologise if this has been asked numerous times before!
I am interested in the following adiabatic system, I think it counts as an isolated system:
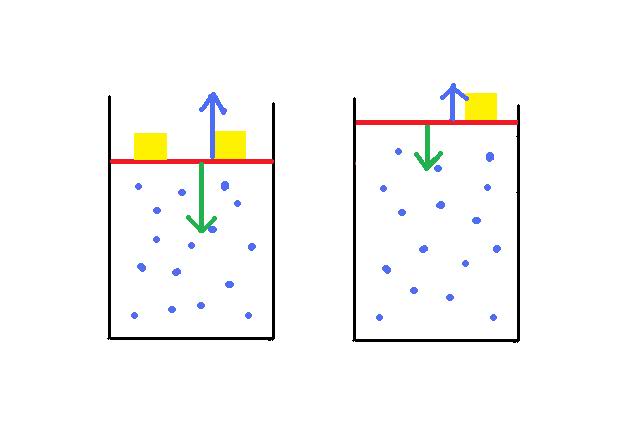
Here is my attempt to explain what is happening:
The internal energy of this system decreases when I remove one of the weights (gold) on the lid (red). I was wondering why this is so. The change in the internal energy is a result of either heat being trasnferred or introduced, or work being done by or to the system. In this case, heat is not a factor, so it must be solely due to work. I can see that work is being by the system. But I am interested in the 'factors' involved.
The lid pushes down on the system due to gravity (green arrow). The gas pushes back with a net force that is due to the particles each exerting a force on the lid by bumping into it. The lid does not go down or up as the two forces are equal. I assume that the kinteic energy of the system is constant because the energy a particle transfers to the lid is 'recouped' by the lids collision with the particle?! I ask this, becasue it ties in with the next bit.
If I remove a gold weight, the force exerted by the lid is reduced, and the particles push against the lid. They transfer kinetic energy to the lid (and gold weight), this kinetic energy is then transformed into gravitational potential energy as the lid moves up. This happens until the particles loose enough kinetic energy in moving the lid (by transferring their kinetic energy to the lid) AND because the volume is larger that the particles collide with the lid less often (reducing the force they exert), that the force exerted by the lids new weight due to gravity is equal to the new kinetic energy in the system.
Is this the correct interpretation?
Any help appreciated.
I'm not sure if this should be in the physics section or chemistry, but I think it's covered in both. I apologise if this has been asked numerous times before!
I am interested in the following adiabatic system, I think it counts as an isolated system:
Here is my attempt to explain what is happening:
The internal energy of this system decreases when I remove one of the weights (gold) on the lid (red). I was wondering why this is so. The change in the internal energy is a result of either heat being trasnferred or introduced, or work being done by or to the system. In this case, heat is not a factor, so it must be solely due to work. I can see that work is being by the system. But I am interested in the 'factors' involved.
The lid pushes down on the system due to gravity (green arrow). The gas pushes back with a net force that is due to the particles each exerting a force on the lid by bumping into it. The lid does not go down or up as the two forces are equal. I assume that the kinteic energy of the system is constant because the energy a particle transfers to the lid is 'recouped' by the lids collision with the particle?! I ask this, becasue it ties in with the next bit.
If I remove a gold weight, the force exerted by the lid is reduced, and the particles push against the lid. They transfer kinetic energy to the lid (and gold weight), this kinetic energy is then transformed into gravitational potential energy as the lid moves up. This happens until the particles loose enough kinetic energy in moving the lid (by transferring their kinetic energy to the lid) AND because the volume is larger that the particles collide with the lid less often (reducing the force they exert), that the force exerted by the lids new weight due to gravity is equal to the new kinetic energy in the system.
Is this the correct interpretation?
Any help appreciated.