- #1
MSM
- 12
- 0
- TL;DR Summary
- Trying to understand how to approximate the time it takes for a fluid to reach the heated tube temperature
Hi,
I am trying to understand how I can estimate the time it takes for a fluid at room temperature flowing through a thin capillary glass tube (2 mm ID) connected to an oven to reach the equilibrium temperature (oven temperature). Assuming the oven is preheated and the tube inside the oven is equilibrated to the oven temperature (300 C)
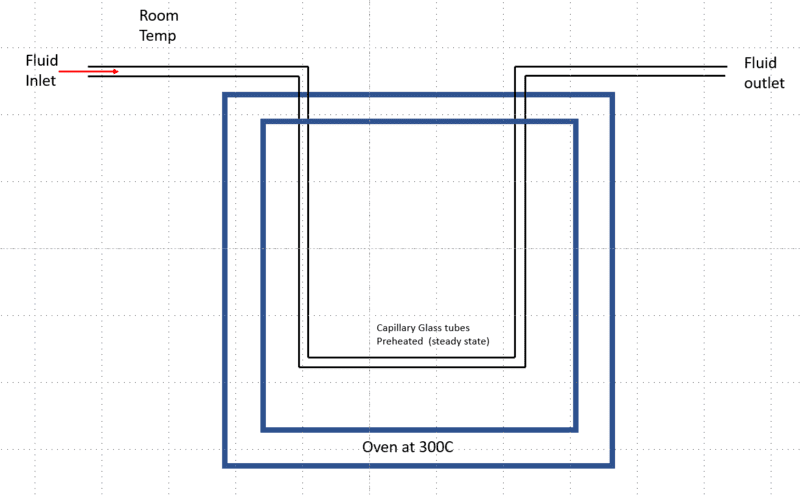
What I did was I assumed lumped capacitance model approximation and calculated the time. My understanding this would be a transient heat conduction problem, and this would be considered a constant flux since the tube inside the oven is already equilibrated to oven temperature before the fluid entering. Since this is a millimeter scale tube, I expected a millisecond time scale for fluid to reach oven temperature, but I got something in the 20+ second range What am I missing? I am only interested in an approximation, not exact numbers.
I am trying to understand how I can estimate the time it takes for a fluid at room temperature flowing through a thin capillary glass tube (2 mm ID) connected to an oven to reach the equilibrium temperature (oven temperature). Assuming the oven is preheated and the tube inside the oven is equilibrated to the oven temperature (300 C)
What I did was I assumed lumped capacitance model approximation and calculated the time. My understanding this would be a transient heat conduction problem, and this would be considered a constant flux since the tube inside the oven is already equilibrated to oven temperature before the fluid entering. Since this is a millimeter scale tube, I expected a millisecond time scale for fluid to reach oven temperature, but I got something in the 20+ second range What am I missing? I am only interested in an approximation, not exact numbers.