Carlos_Ishigami07
- 10
- 2
- TL;DR Summary
- Applications
They know what this equation or formula and what applications it has. I saw in the anime Dr. Stone.
Thanks for your understanding.
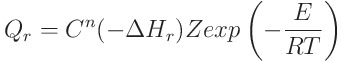
Thanks for your understanding.
Last edited by a moderator: