Zayn
- 9
- 0
Homework Statement
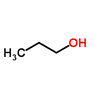
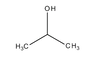
Why is the boiling point of 2-propanol lower than 1-propanol?
The Attempt at a Solution
Is this right?
Because 2-propanol has its hydroxyl group in the middle of the atom, the electrons are all moving to the centre of the atom as opposed to 1-propanol, which has the hydroxyl group to the side, moving the electrons towards one extreme of the molecule, causing it to act similarly to a magnet. Because 1-propanol has its hydroxyl to one side, the hydrogen bonds it forms can pack together more densely than with 2-propanol, requiring more kinetic energy (temperature) to break them apart, which translates to a higher boiling point.