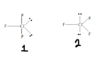
ClF3 exhibits a trigonal bipyramidal arrangement with a T-shaped molecular geometry due to the presence of non-bonding electron pairs that exert greater steric repulsion compared to bonding pairs. In VSEPR theory, non-bonding pairs are considered sterically more demanding, meaning they require more space and occupy a larger volume, which influences molecular shape. The discussion highlights that in the first case, non-bonding pairs are positioned with only two neighbors at 90-degree angles, while in the second scenario, they would have three neighbors at 120 degrees, leading to increased repulsion. This repulsion is crucial in determining the actual geometry of ClF3, reinforcing why it does not adopt the second case's trigonal planar shape. The term "sterically more demanding" refers to the need for non-bonding pairs to have more space due to their larger volume compared to bonding pairs.