- #1
DaTario
- 1,039
- 35
- TL;DR Summary
- Hi, All, I would like to know the following figure is ok in the context of explaining to beginers the theory of bands (solid state physics).
Hi, All. In searching for images related to the introduction of band theory in solid sate physics I found this one:
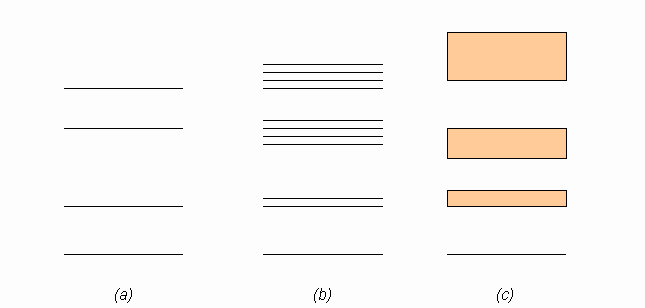
I would like to ask you if I am correct in thinking the increase in the number of splittings (in b) is misleading to students.
Thank you all
DaTario
I would like to ask you if I am correct in thinking the increase in the number of splittings (in b) is misleading to students.
Thank you all
DaTario
Last edited: