themonk
- 16
- 0
I understand the rules of aromaticity, but I do not understand why Tropolone is aromatic. The main reason is because I thought all the atoms in the ring have to have p-orbitals. How does the C connected the oxygen with a double bond have a p-orbital? Have to do with the resonance (that's my main guess)?
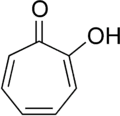
It passes Hückel's rule with 6e- pi electrons and also passes other criteria.
It passes Hückel's rule with 6e- pi electrons and also passes other criteria.