Good4you
- 37
- 0
I use a C02 tank to fill up the tires on my truck. C02 is used because it can store a larger volume of gas in a smaller tank than most other gases. But i have no idea why this would be true. I can actually fill all four 35" tires about four times flat to full with one 10# tank.
Nitrogen would be better because it could also charge my shocks, but from what i understand it would not hold as much. Also, i have heard that scuba tanks have to use insanely high pressures to keep a decent amount of air.
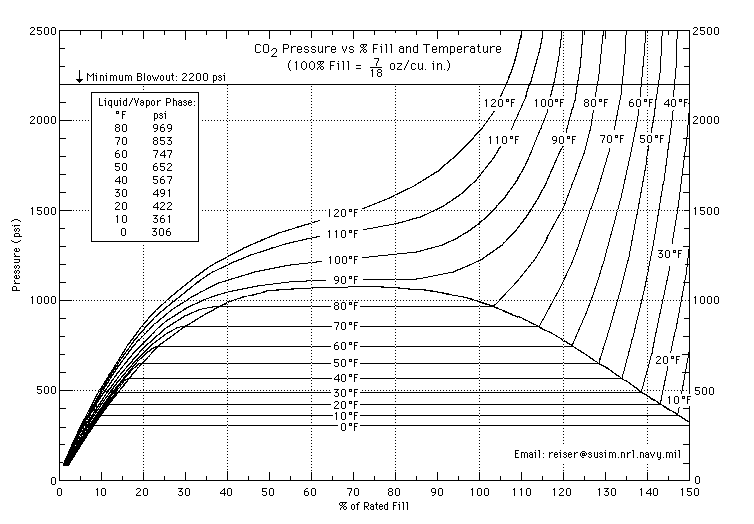
One reason i have heard is that C02 keeps a constant pressure in the tank regardless of how full it is, and the liquid C02 just boils off. And this seems to be true from the chart below. But wouldn't this violate Boyle's law?
Also, i assume Nitrogen or air isn't used because it takes more pressure to turn to a liquid at room temperature. Is that true?
Nitrogen would be better because it could also charge my shocks, but from what i understand it would not hold as much. Also, i have heard that scuba tanks have to use insanely high pressures to keep a decent amount of air.
One reason i have heard is that C02 keeps a constant pressure in the tank regardless of how full it is, and the liquid C02 just boils off. And this seems to be true from the chart below. But wouldn't this violate Boyle's law?
Also, i assume Nitrogen or air isn't used because it takes more pressure to turn to a liquid at room temperature. Is that true?