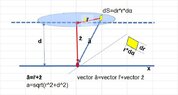
- #1
falyusuf
- 35
- 3
- Homework Statement
- Attached below.
- Relevant Equations
- Attached below.
Question
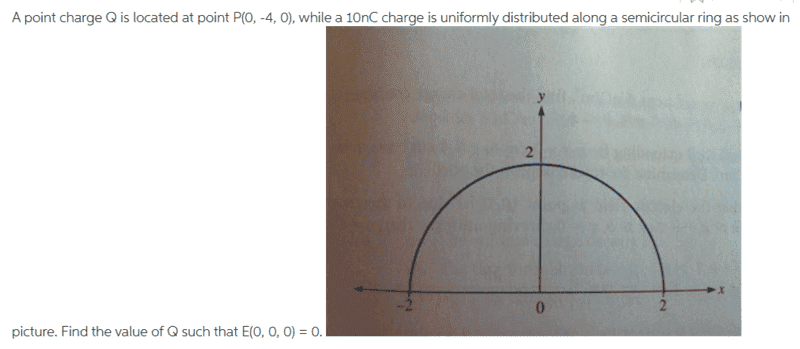
Relevant equation;
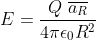
My attempt:
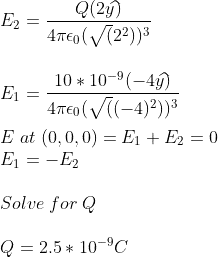
Could someone please confirm my answer?
Relevant equation;
My attempt:
Could someone please confirm my answer?