Kushwoho44
- 25
- 1
Hi there, I hold an engineering degree and I was just reviewing a page on Wikipedia.
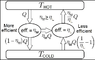
This image specifically demonstrates the impossibility of two theoretical heat engines having different efficiencies between two heat reservoirs. The full Wikipedia page can be found: https://en.wikipedia.org/wiki/Carnot's_theorem_(thermodynamics)
The terms in green I could arrive at and they confirm my understanding.
The terms in red have confused me. If I can get one of the terms, I can necessarily deduce the other and then it becomes clear to me why this cycle is impossible.
However, without knowing either of these terms, I can't understand how they have arrived at one of them alone. I suspect that they have deduced the ηm/ηl Q term from the definition of efficiency. Herein is the knot in my understanding. Why would a definition of efficiency not include a total ratio of all the energy entering the heat pump and all the energy leaving the system? I cannot understand why the definition of efficiency does not include the energy coming into the system from the cold reservoir?
Kind regards.