- #1
demander
- 26
- 0
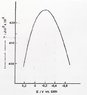
this image was given as the electrocapillary curve for mercury
http://img41.imageshack.us/img41/1083/electroo.th.jpg
for a solution of 0,1M KCL
they ask what is the excess of charge density at 0,1V
So i Know that i need to use the Lippmann equation
[tex]{\sigma}_M=\frac{\partial\gamma}{\partial E(V)}[/tex]
but as i don't have any expression the only thing given is the graphic and the 0,1V that's the point we want, I'm really stuck
i tried to solve it but seems that my problem is that i can't find the way to make this, a teacher said that by the maths way(tangent method) we can determinate the value at 0,1V as the Lippmann equation is purelly a derivative of the graphic, if someone knows how to derivate the graph without any expression, using only things taken from graphic i would thank
i think that's the way to do, don't really know if is really this way
so as said the only data given is the graphic values, the usage os Lippmann equation written above, the concentration 0,1M of KCl but this only a indication don't thinks is really needed and the indication that we want the excess charge density at 0,1V
i hope you can help me, if there is something near indicate-me the link or move to the right section, i think my problem is more a maths problem than chemistry, but I'm confused
if the image don't work i put them in attachement to
http://img41.imageshack.us/img41/1083/electroo.th.jpg
for a solution of 0,1M KCL
they ask what is the excess of charge density at 0,1V
So i Know that i need to use the Lippmann equation
[tex]{\sigma}_M=\frac{\partial\gamma}{\partial E(V)}[/tex]
but as i don't have any expression the only thing given is the graphic and the 0,1V that's the point we want, I'm really stuck
i tried to solve it but seems that my problem is that i can't find the way to make this, a teacher said that by the maths way(tangent method) we can determinate the value at 0,1V as the Lippmann equation is purelly a derivative of the graphic, if someone knows how to derivate the graph without any expression, using only things taken from graphic i would thank
i think that's the way to do, don't really know if is really this way
so as said the only data given is the graphic values, the usage os Lippmann equation written above, the concentration 0,1M of KCl but this only a indication don't thinks is really needed and the indication that we want the excess charge density at 0,1V
i hope you can help me, if there is something near indicate-me the link or move to the right section, i think my problem is more a maths problem than chemistry, but I'm confused
if the image don't work i put them in attachement to
Attachments
Last edited by a moderator: