sam.
- 16
- 0
Homework Statement
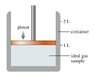
This figure (file attached) shows a container that is sealed at the top by a movable piston. Inside the container is a ideal gas at 1.00 atm, 20.0 degrees Celsius, and 1.00 L.
The gas sample has now returned to its original state of 1.00 atm, 20.0 degrees Celsius and 1.00 L. What will the pressure become if the temperature of the gas is raised to 200.0degrees celsius and the piston is not allowed to move?
Homework Equations
Boyle's Law P_1V_1 = P_2V_2
or
Charles' Law V_1/T_1 = V_2/T_2
The Attempt at a Solution
Okay, in order for the piston not to move, this means that the volume must remain at 1.00 L. What I don't know what to do is how to relate temperature and pressure to solve for the final pressure.