chemaster
- 5
- 4
Hello,
I will be conducting an experiment to create a sugar monocrystal, the device I will be using (apologies, English is not my primary language therefore I do not know all the proper terms) will be modeled and influenced by the one described in the paper "Soft-sensor for industrial sugar crystallization: On-line mass of crystals, concentration and purity measurement" by Cedric Damour, Michel Benne, Brigitte Grondin-Perez and Jean-Pierre Chabriat.
I am currently attempting to contact them, but would like some additional input from other professionals.
Schema of the device:
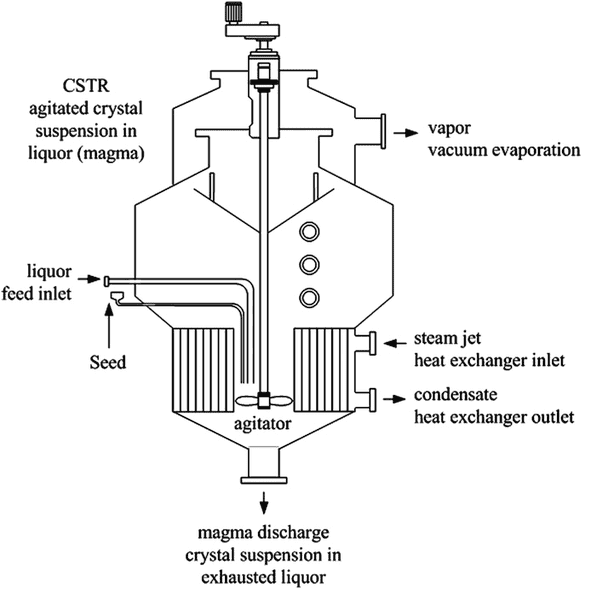
The gist of the entire setup is that in order to gain a sugar monocrystal you need to have a highly saturated solution heated to a certain temperature and very slowly cool it down, in the range of one degree per day.
The tasks this device/experimental setup needs to accomplish are the following:
1. Have a pool of the sugar solution, amidst which a seed crystal may or may not rest. (the problem with the placement of a seed crystal being that I am looking to obtain a near-perfect result and I cannot figure out how to place a seedling without any sort of support)
2. Keep the solution at a certain temperature that can be finely controlled.
3. Vent vapors from the sugar solution.
4. Prevent dust and other polluting particles to enter the chamber.
5. Gently stir the solution in order to prevent differing gradient concentration.
6. Circulate the solution so that fresh solution may enter the chamber.
So far this is what I am thinking and the first problems I can think of:
1. Designing the sealed metallic chamber - what dimensions should it have / are optimal? Does the material matter?
2. Should I think of anything else for the heating besides external heating elements (or internal, but then I would have to consider how they will interfere in the crystal growth) that are insulated and can be fine controlled with a display of the current temperature in the tank?
3. What should be considered for the liquid injet so that it doesn't clog or develop crystals of its own?
4. Since I prefer for the stirring to place a propeller-similar device at the bottom of the tank and let it rotate automatically with an angular velocity yet to be decided, are there better options?
5. How should I vent the solution vapors effectively while at the same time not influencing the crystal growth OR letting in pollution particles?
Thank you for your time!
I will be conducting an experiment to create a sugar monocrystal, the device I will be using (apologies, English is not my primary language therefore I do not know all the proper terms) will be modeled and influenced by the one described in the paper "Soft-sensor for industrial sugar crystallization: On-line mass of crystals, concentration and purity measurement" by Cedric Damour, Michel Benne, Brigitte Grondin-Perez and Jean-Pierre Chabriat.
I am currently attempting to contact them, but would like some additional input from other professionals.
Schema of the device:
The gist of the entire setup is that in order to gain a sugar monocrystal you need to have a highly saturated solution heated to a certain temperature and very slowly cool it down, in the range of one degree per day.
The tasks this device/experimental setup needs to accomplish are the following:
1. Have a pool of the sugar solution, amidst which a seed crystal may or may not rest. (the problem with the placement of a seed crystal being that I am looking to obtain a near-perfect result and I cannot figure out how to place a seedling without any sort of support)
2. Keep the solution at a certain temperature that can be finely controlled.
3. Vent vapors from the sugar solution.
4. Prevent dust and other polluting particles to enter the chamber.
5. Gently stir the solution in order to prevent differing gradient concentration.
6. Circulate the solution so that fresh solution may enter the chamber.
So far this is what I am thinking and the first problems I can think of:
1. Designing the sealed metallic chamber - what dimensions should it have / are optimal? Does the material matter?
2. Should I think of anything else for the heating besides external heating elements (or internal, but then I would have to consider how they will interfere in the crystal growth) that are insulated and can be fine controlled with a display of the current temperature in the tank?
3. What should be considered for the liquid injet so that it doesn't clog or develop crystals of its own?
4. Since I prefer for the stirring to place a propeller-similar device at the bottom of the tank and let it rotate automatically with an angular velocity yet to be decided, are there better options?
5. How should I vent the solution vapors effectively while at the same time not influencing the crystal growth OR letting in pollution particles?
Thank you for your time!