phrendlie
- 2
- 0
I would like to know what the dots are. This reminds me of X-ray diffraction, but I am imaging a sphere of liquid on the end of a microcapillary nozzle (2um inner diameter glass nozzle with sputtered gold outer coating on nozzle).
I am taking a picture of a small glass nozzle with a metal coating. There is a sphere of liquid at the end of the nozzle This sphere has radius ~10 to 20 um. Then I lowered the lights and took a long exposure (1 second). The lighting is a single red LED, with some overhead white lights.
The LED hits the sphere of liquid at ~120 degrees to the camera with the light coming from behind the object. By this, I mean that the light has to bend ~60 degrees within the sphere to reach the camera. The LED light is pulsing at 10 Hz.
Low light, long exposure (1 second) with overhead lights on:

Same, with overhead lights off:

Then I put a mirrored surface under the nozzle to make the image brighter (requiring shorter exposure time)
Exposure time 8 ms, no change in focus or magnification, no change in light, camera, or object position. Overhead lights off, so all light is red, nearly monochromatic:
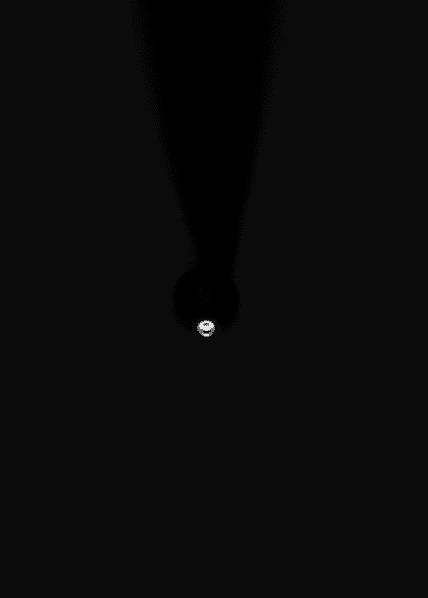
I am taking a picture of a small glass nozzle with a metal coating. There is a sphere of liquid at the end of the nozzle This sphere has radius ~10 to 20 um. Then I lowered the lights and took a long exposure (1 second). The lighting is a single red LED, with some overhead white lights.
The LED hits the sphere of liquid at ~120 degrees to the camera with the light coming from behind the object. By this, I mean that the light has to bend ~60 degrees within the sphere to reach the camera. The LED light is pulsing at 10 Hz.
Low light, long exposure (1 second) with overhead lights on:
Same, with overhead lights off:
Then I put a mirrored surface under the nozzle to make the image brighter (requiring shorter exposure time)
Exposure time 8 ms, no change in focus or magnification, no change in light, camera, or object position. Overhead lights off, so all light is red, nearly monochromatic: