- #1
Wminus
- 173
- 29
Hi. Here's the dispersion relation for a diatomic linear chain, where the distance is a/2 between each atom.
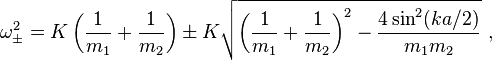
My issue here is that if you set m_1=m_2=m, i.e. set both atoms equal to each other, it doesn't automatically reduce to the old acoustic dispersion relation as the ± term doesn't disappear.
What's up with that?
My issue here is that if you set m_1=m_2=m, i.e. set both atoms equal to each other, it doesn't automatically reduce to the old acoustic dispersion relation as the ± term doesn't disappear.
What's up with that?