Telemachus
- 820
- 30
Hi there, I have to solve this problem:
Use the following data to estimate the molarity of a saturated aqueous solution of ##Sr(IO_3)_2##
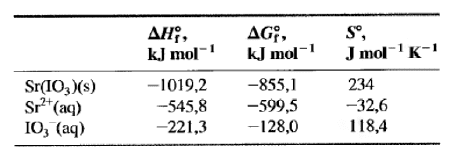
So, I think I should use the Van't Hoff equation in some way, but I don't know how.
I also have:
##\Delta_r G=\Delta G^o+RT\ln K##
##K## is the equilibrium constant, and ##\Delta G^o## is the Gibbs energy of formation.
In equilibrium ##\Delta_r G=0## and the equation can be managed to get the Van't Hoff equation, which is:
##\ln K_1-\ln K_2=-\displaystyle\frac{\Delta H^o}{R} \left( \displaystyle\frac{1}{T_2}-\displaystyle\frac{1}{T_1} \right)##
I think that I should handle this equations to get the equilibrium constant in some way, and then the molarity. Another equation that may be useful is the definition of the Gibbs energy:
##\Delta G^o=\Delta H^o-T\Delta S^o##
The chemical equation involved I think should be:
##Sr(IO_3)_2(s)+H_2O(l) \rightleftharpoons Sr^{2+}(aq)+2IO_3^{-}##
And from it: ##K'=\displaystyle\frac{[Sr^{2+}][IO_3^{-}]^2}{[Sr(IO_3)_2]}##
The solid concentration remains constant, and then: ##K=[Sr^{2+}][IO_3^{-}]^2##
Can anybody help me to work this out?
Thanks.
Use the following data to estimate the molarity of a saturated aqueous solution of ##Sr(IO_3)_2##
So, I think I should use the Van't Hoff equation in some way, but I don't know how.
I also have:
##\Delta_r G=\Delta G^o+RT\ln K##
##K## is the equilibrium constant, and ##\Delta G^o## is the Gibbs energy of formation.
In equilibrium ##\Delta_r G=0## and the equation can be managed to get the Van't Hoff equation, which is:
##\ln K_1-\ln K_2=-\displaystyle\frac{\Delta H^o}{R} \left( \displaystyle\frac{1}{T_2}-\displaystyle\frac{1}{T_1} \right)##
I think that I should handle this equations to get the equilibrium constant in some way, and then the molarity. Another equation that may be useful is the definition of the Gibbs energy:
##\Delta G^o=\Delta H^o-T\Delta S^o##
The chemical equation involved I think should be:
##Sr(IO_3)_2(s)+H_2O(l) \rightleftharpoons Sr^{2+}(aq)+2IO_3^{-}##
And from it: ##K'=\displaystyle\frac{[Sr^{2+}][IO_3^{-}]^2}{[Sr(IO_3)_2]}##
The solid concentration remains constant, and then: ##K=[Sr^{2+}][IO_3^{-}]^2##
Can anybody help me to work this out?
Thanks.
Attachments
Last edited: