maxpowers_00
- 5
- 0
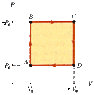
An ideal gas initially at Pi, Vi, and Ti is taken through a cycle as in Figure P20.38 (n = 2.6).
Figure P20.38 (see attachment)
(a) Find the net work done on the gas per cycle.
PiVi
(b) What is the net energy added by heat to the system per cycle?
PiVi
(c) Obtain a numerical value for the net work done per cycle for 1.15 mol of gas initially at 0°C.
kJ
a) i though at first that W= area of the cycle, which came out to be 6.76 but, it was wrong.
as for part b, i have no idea
c) W=nRTln(vi/2.6vi) = W=(1.15)(8.314)(273)ln(1/2.6) = 2.5kJ but this also turned out to be wrong
any ideas?
thanks
Figure P20.38 (see attachment)
(a) Find the net work done on the gas per cycle.
PiVi
(b) What is the net energy added by heat to the system per cycle?
PiVi
(c) Obtain a numerical value for the net work done per cycle for 1.15 mol of gas initially at 0°C.
kJ
a) i though at first that W= area of the cycle, which came out to be 6.76 but, it was wrong.
as for part b, i have no idea
c) W=nRTln(vi/2.6vi) = W=(1.15)(8.314)(273)ln(1/2.6) = 2.5kJ but this also turned out to be wrong
any ideas?
thanks