- #1
rachelmaddiee
- 67
- 5
- Homework Statement
- I need help with a question
- Relevant Equations
- N/A
Here is my work**
Magnesium cations (Mg2+)
Oxide anions (O 2-)
A magnesium ion has a 2+ charge and the oxygen atom has a 2- charge. The formula for the ionic compound is MgO.
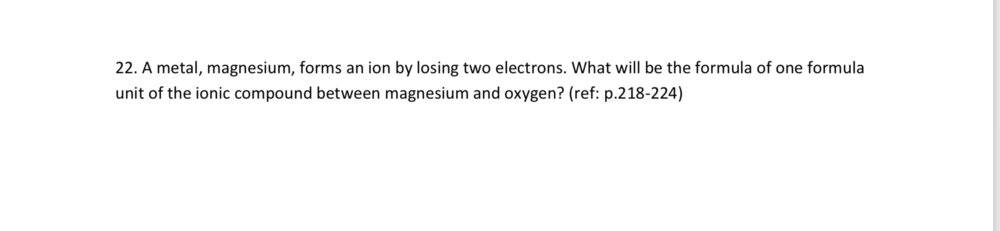
Magnesium cations (Mg2+)
Oxide anions (O 2-)
A magnesium ion has a 2+ charge and the oxygen atom has a 2- charge. The formula for the ionic compound is MgO.