Sirsh
- 262
- 10
I have a thermodynamic question which has been answered by my lecturer in a qualitative rather than a quantitative fashion.
The question is:
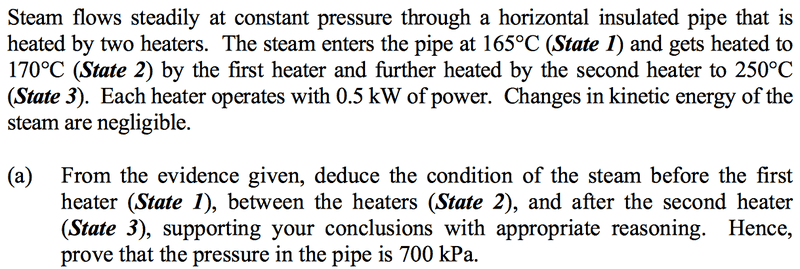
My question is can this be answered in a quantitative fashion? I've been trying to find a way but to no avail.
My thought process is such that using the SFEE you could some how find a difference of internal energy between two of the three temperatures within the steam tables. But I cannot figure out how to do this explicitly, due to the fact the heat energy supplied is so small and that no other information is given such as masses etc..
Thanks!
The question is:
My question is can this be answered in a quantitative fashion? I've been trying to find a way but to no avail.
My thought process is such that using the SFEE you could some how find a difference of internal energy between two of the three temperatures within the steam tables. But I cannot figure out how to do this explicitly, due to the fact the heat energy supplied is so small and that no other information is given such as masses etc..
Thanks!