tomdodd4598
- 137
- 13
Hey there - I think I have an issue with my 3D density plots of the probability density of the Coulomb wave function. The reason I think something is going wrong is because my plots of |ψ(n=2, l=1, m=-1)|² and |ψ(2, 1, 1)|² are identical, while I would expect them to have the same shape but be rotationally symmetric along different orthogonal axes.
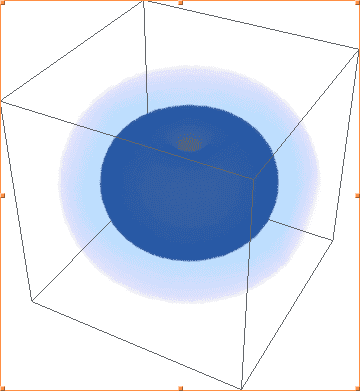
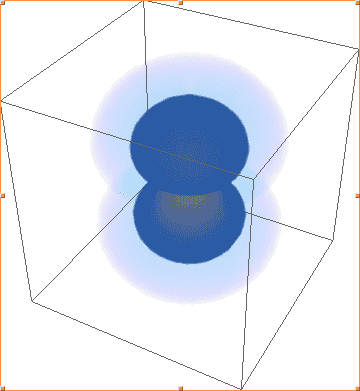
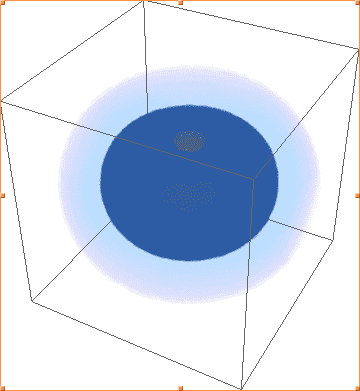
The above images are Mathematica's plots of |ψ(2, 1, -1)|², |ψ(2, 1, 0)|², |ψ(2, 1, 1)|², respectively. As you can see, the first and third are identical, and not the shape of 2p orbitals, while the second plot actually looks like what I would expect - one of the three 2p orbitals.
Here is my wave function - it's possible that the conversion from spherical to Cartesian coordinates is a problem, but I'm not sure:

If the above needs clarifying, do ask. Thanks for any help in advance ;)
The above images are Mathematica's plots of |ψ(2, 1, -1)|², |ψ(2, 1, 0)|², |ψ(2, 1, 1)|², respectively. As you can see, the first and third are identical, and not the shape of 2p orbitals, while the second plot actually looks like what I would expect - one of the three 2p orbitals.
Here is my wave function - it's possible that the conversion from spherical to Cartesian coordinates is a problem, but I'm not sure:
If the above needs clarifying, do ask. Thanks for any help in advance ;)