- #1
TomK
- 69
- 14
- Homework Statement
- ENGAA 2019 (Q8, Section 2)
- Relevant Equations
- Pressure Equations
Ideal Gas Laws
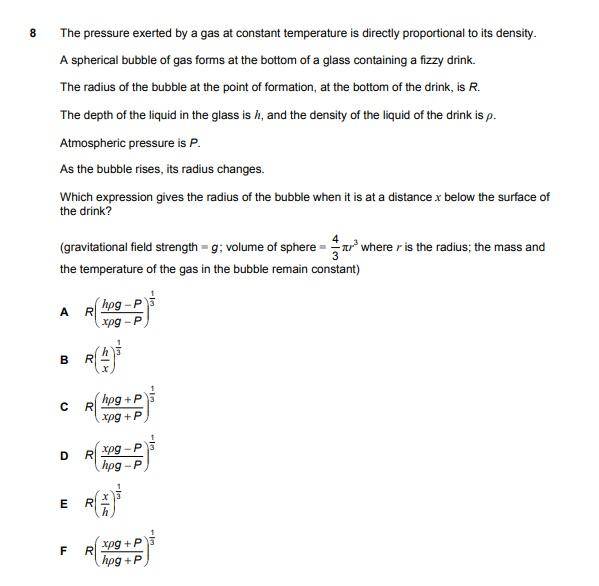
The correct answer is 'C'.
I'm having trouble understanding the solution shown on this link (http://www.engineeringadmissionsassessment.com/2019-solutions.html) - scroll down to Section 2, Question 8.
From what I've gathered, [final pressure = initial pressure x 'R^3/r^3'], as PV must be constant. Therefore, I understand why they equate 'R^3/r^3' to 'final pressure/initial pressure'.
However, why doesn't the pressure exerted by the gas (within the bubble) affect the values of the initial and final pressure, since the bubble's volume is changing as it rises? Doesn't the pressure exerted by the gas (outward) oppose the pressure exerted on the gas (inward) by the liquid and atmospheric pressure?
In the solution, it looks like their initial/final pressure values only account for the 'pressure due to being submerged' and 'atmospheric pressure'. This is what I'm getting confused by.