- #1
cwill53
- 220
- 40
- Homework Statement
- Using a pressurized line, a fluid is charged in a rigid tank with volume ##2m^3##. Derive the expression and calculate:
1. The internal energy ##u_t## of the fluid in that tank supposing the initial mass is ##m_0=0## , and specific enthalpy ##h_i = 1670 kJ/kg.##
2. Assuming a fluid is an ideal gas, derive and calculate the tank temperature using the specific heat ratio of k = 1.67 and the initial temperature of ##T_i=400 ^{\circ}C##
3. Assuming a fluid obeys ideal gas law and a flow is choked derive and calculate the tank pressure as a function of time using ##\dot{m}=0.5kg/s,t=2s,k=1.67, T_i=400^{\circ}C##
Assume that charging occurs over a short period of time.
- Relevant Equations
- $$pV\propto RT$$
$$H=U+pV$$
$$dU=\delta Q-\delta W$$
I'm stuck on part 1 at the moment. What I had so far was
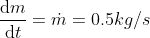
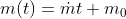
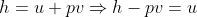
Using the ideal gas model, we have
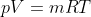
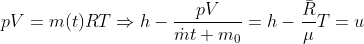
Solving these equations is reliant on the molar mass μ of the substance or the pressure p in the tank, which I don't know how to find with the given information. I sent an email to my professor and he told me to start with the first law of thermodynamics, cancel out appropriate terms and then use mass conservation taking into account that initial mass is zero. I'm not sure how to solve for the specific internal energy if that's the case. This system is isochoric if I'm not mistaken, does this mean the specific internal energy is equal to the initial specific enthalpy ##h_i=1670kJ/kg## or am I over thinking this and the specific internal energy ##u_t## is actually zero because the initial mass is zero?
Using the ideal gas model, we have
Solving these equations is reliant on the molar mass μ of the substance or the pressure p in the tank, which I don't know how to find with the given information. I sent an email to my professor and he told me to start with the first law of thermodynamics, cancel out appropriate terms and then use mass conservation taking into account that initial mass is zero. I'm not sure how to solve for the specific internal energy if that's the case. This system is isochoric if I'm not mistaken, does this mean the specific internal energy is equal to the initial specific enthalpy ##h_i=1670kJ/kg## or am I over thinking this and the specific internal energy ##u_t## is actually zero because the initial mass is zero?