lemonbrook
- 2
- 0
Hi all,
1. The problem statement
A copper specimen, initially at 293K, is immersed in a bath of liquid salt at 773K. The bath is agitated and the heat transfer at the surface of the metal is effectively perfect. A temperature of 723K is measured at 5mm below the surface of the metal item after 3 seconds. Estimate the depth below the surface at which a temperature of 723K would be reached in 3 seconds if the specimen were made of titanium alloy.
Copper
Thermal Conductivity = 380 W m-1 K-1
Specific Heat Capacity = 390 J kg-1 K-1
Density - 8900 kg m-3
Titanium
Thermal Conductivity = 6 W m-1 K-1
Specific Heat Capacity = 500 J kg-1 K-1
Density - 4500 kg m-3
This equation wasn't given in the question but seems to be relevant.
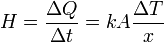
where
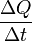 is the rate of heat flow, k is the thermal conductivity, A is the total cross sectional area of conducting surface, ΔT is temperature difference, and x is the thickness of conducting surface separating the 2 temperatures.
is the rate of heat flow, k is the thermal conductivity, A is the total cross sectional area of conducting surface, ΔT is temperature difference, and x is the thickness of conducting surface separating the 2 temperatures.
I'm very confused as to how to approach this problem. I first attempted to rearrange the equation for Area, but I would also need to have ΔQ which would require a mass to calculate. Could I just use and arbitrary volume for both components and use the density to calculate a mass?
Thanks
1. The problem statement
A copper specimen, initially at 293K, is immersed in a bath of liquid salt at 773K. The bath is agitated and the heat transfer at the surface of the metal is effectively perfect. A temperature of 723K is measured at 5mm below the surface of the metal item after 3 seconds. Estimate the depth below the surface at which a temperature of 723K would be reached in 3 seconds if the specimen were made of titanium alloy.
Copper
Thermal Conductivity = 380 W m-1 K-1
Specific Heat Capacity = 390 J kg-1 K-1
Density - 8900 kg m-3
Titanium
Thermal Conductivity = 6 W m-1 K-1
Specific Heat Capacity = 500 J kg-1 K-1
Density - 4500 kg m-3
Homework Equations
This equation wasn't given in the question but seems to be relevant.
where
The Attempt at a Solution
I'm very confused as to how to approach this problem. I first attempted to rearrange the equation for Area, but I would also need to have ΔQ which would require a mass to calculate. Could I just use and arbitrary volume for both components and use the density to calculate a mass?
Thanks