JHUK
- 5
- 0
I posted in picture format to post on another website, but haven't found a reply yet:
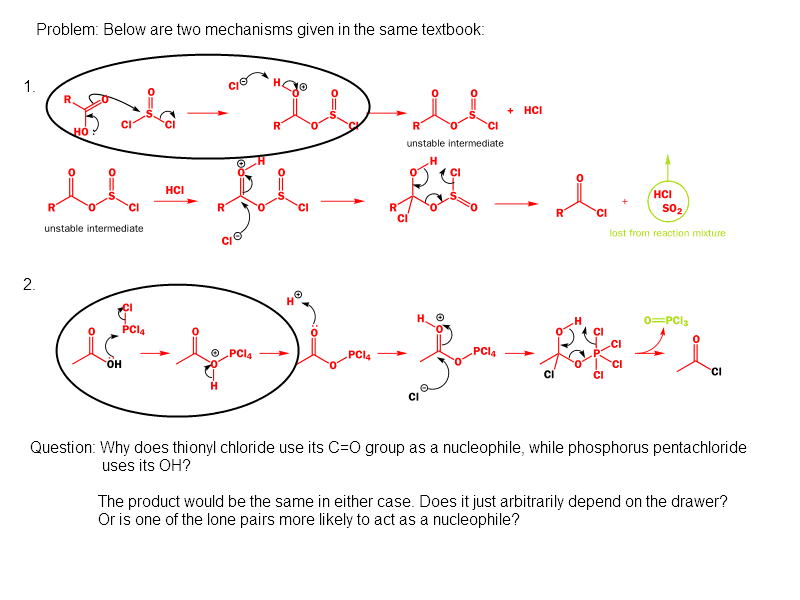
Yanick said:You're question is a little unclear because the mechanism shows nucleophilic attack by a -COOH group. The oxygens in these groups are actually equivalent and are best represented by resonance structures where the hydroxyl and carbonyl oxygens exchange.
sjb-2812 said:No, they are equivalent in the carboxylate anion, but not in the protonated version. Resonance structures do not involve the movement of protons.