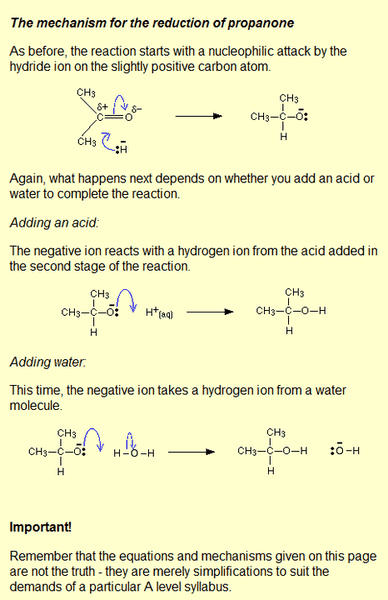
The discussion focuses on the simplifications present in the explanation of nucleophilic addition reactions, particularly regarding the reduction of carbonyl compounds. Key points include the recognition that many reactions are equilibrium processes, with reversible steps such as the formation of tetrahedral intermediates. It is emphasized that hydride ions do not exist freely in solution but are derived from reducing agents like lithium aluminum hydride or sodium borohydride, which also influence the reaction's mechanism through stabilization of intermediates. The choice of solvents is crucial, as different solvents can affect the formation of intermediates. The conversation also touches on the importance of understanding simplified models in organic chemistry, noting that while these models may not capture the full complexity of reactions, they provide valuable insights into reaction mechanisms and barriers. Additionally, the discussion highlights the balance between using valence bond theory and molecular orbital theory for understanding chemical behavior, with a consensus on the utility of simplified models in educational contexts.