brendan714
- 5
- 0
So I wrote my Thermodynamics final today and one of the questions were as follows:
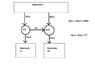
A reversible heat engine supplies a reversible heat pump. Both draw off of a temperature reservoir T (can't remember the exact value, but there was one). The heat engine discharges heat into TL, and the heat pump discharges heat into TH.
You know that QH for the Heat Engine (heat drawing from reservoir T) and QL for the Heat Pump (heat drawing from reservoir T) together equal 5 MW.
What is the total amount of heat discharged by the Heat Engine and the Heat Pump into their respective reservoirs?
I just simply said that the answer must be 5 MW since both are reversible (no losses anywhere)! Does anyone agree/think I am wrong?
Thanks!
A reversible heat engine supplies a reversible heat pump. Both draw off of a temperature reservoir T (can't remember the exact value, but there was one). The heat engine discharges heat into TL, and the heat pump discharges heat into TH.
You know that QH for the Heat Engine (heat drawing from reservoir T) and QL for the Heat Pump (heat drawing from reservoir T) together equal 5 MW.
What is the total amount of heat discharged by the Heat Engine and the Heat Pump into their respective reservoirs?
I just simply said that the answer must be 5 MW since both are reversible (no losses anywhere)! Does anyone agree/think I am wrong?
Thanks!