You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Where is the work done coming from in Helmholtz free energy
- Thread starter tze liu
- Start date
AI Thread Summary
The discussion centers on the concept of work done in thermodynamic systems, particularly when the volume is fixed. It clarifies that while no P-V work occurs under constant volume conditions, other forms of work, such as electrochemical work or stirring, can still take place without changing the volume. The Helmholtz free energy is highlighted as a measure of a system's capacity to perform work, even when the system is thermally isolated. The conversation also addresses the confusion surrounding the relationship between temperature in the reservoir and within the system during non-equilibrium processes. Ultimately, the discussion emphasizes that understanding the nuances of free energy and work types is crucial for grasping thermodynamic principles.
Physics news on Phys.org
- 8,943
- 2,954
That description is a little confusing, and the Wikipedia article on the topic is no better.
The article goes on to say that \Delta A can be nonzero because the full definition is dA = -S dt - P dV + \mu dN, where N is the number of particles, and \mu is the chemical potential. So changing the number of particles can also change A. That's true, but doesn't really address the seeming contradiction, as far as I can see.
But here's the way that I think of the various kinds of "free energy". In the first place, imagine that you have two systems that are thermally isolated (no heat in or out) but share the same volume, as in figure 1:
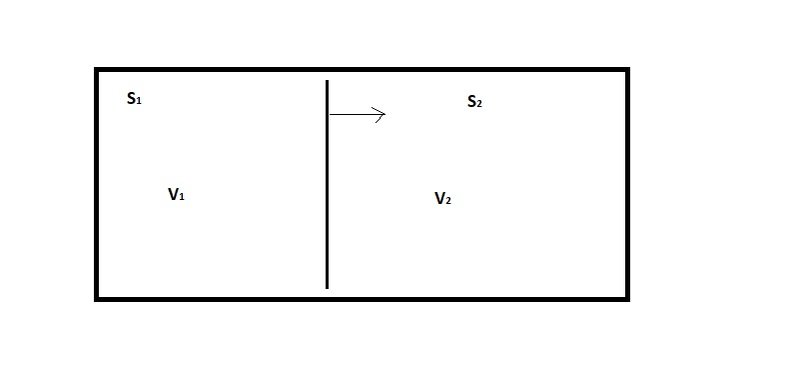
Figure 1: Thermally isolated systems sharing a volume
If system S_1 expands by pushing the partition to the right, then it will do work on system S_2. The amount of work done is given by:
\Delta W = P \Delta V_1
We can use the definition of internal energy to relate the work to the heat going in or out:
\Delta U = \Delta Q - \Delta W = T \Delta S - P \Delta V
(Sometimes this is written with a plus sign in front of \Delta W[/tex], but that depends on whether you're talking about the work done by the system or the work done on the system. If the changes are reversible, as we're assuming, the two are just the negatives of each other).<br /> <br /> If the systems are thermally isolated, then \Delta Q = T \Delta S = 0, which means that \Delta W = - \Delta U<br /> <br /> So the work done by the system is just equal to the loss of internal energy of the system. So in this scenario, the internal energy is a measure of the ability of the system to do work. <br /> <br /> <b>Note</b>: The above discussion is for a reversible change. In an irreversible change, the work done by the system can be less than -\Delta U. So we really get:<br /> <br /> \Delta W \leq - \Delta U<br /> <br /> Now, let's relax the constraint that the two systems are thermally isolated, and instead assume that they are free to exchange heat with a surrounding medium, as in Figure 2:<br /> <div class="bbImageWrapper js-lbImage" title="Delta-A.jpg" data-src="https://www.physicsforums.com/attachments/delta-a-jpg.207957/" data-lb-sidebar-href="" data-lb-caption-extra-html="" data-single-image="1"> <img src="https://www.physicsforums.com/attachments/delta-a-jpg.207957/" data-url="" class="bbImage" data-zoom-target="1" style="" alt="Delta-A.jpg" title="Delta-A.jpg" width="800" height="503" loading="lazy" decoding="async" /> </div> <br /> <b>Figure 2: Systems may exchange heat with surrounding medium, but temperature is kept constant<br /> </b><br /> In this case, it's no longer necessarily true that \Delta Q = 0 when system 1 expands. As it expands, it can absorb heat from the surrounding heat bath, making \Delta Q &gt; 0. But assuming the heat bath is much larger than either of the two systems, the temperature will remain constant during any reversible change of system 1. So we can use a different thermodynamic identity:<br /> <br /> \Delta A = \Delta U - \Delta (S T) = - P \Delta V - S \Delta T<br /> <br /> In this case, the heat bath is keeping the temperature constant, so \Delta T = 0. So we have:<br /> <br /> \Delta A = -P \Delta V = -\Delta W<br /> <br /> So in this case, it is A that gives a measure of the potential of the system to do work, rather than U.<br /> <br /> Again, this is for a reversible change. For an irreversible change, -\Delta A is an upper bound on the amount of work done by the system:<br /> <br /> \Delta W \leq - \Delta A<br /> <br /> The discussion in Wikipedia is misleading, because of course, a system can't do any work if the volume is not allowed to change. I think that the issue is resolved by assuming that there is more than one system involved. The volume of the composite system doesn't change, but the volumes of the smaller subsystems can change, and Helmholtz free energy is a measure of the capacity of the subsystem to do work.
We see that the total amount of work that can be extracted in an isothermal process is limited by the free energy decrease, and that increasing the free energy in a reversible process requires work to be done on the system. If no work is extracted from the system then
\Delta A \leq 0
and thus for a system kept at constant temperature and volume and not capable of performing electrical or other non-PV work, the total free energy during a spontaneous change can only decrease.
This result seems to contradict the equation dA = -S dT - P dV as keeping T and V constant seems to imply dA = 0 and hence A = constant..
The article goes on to say that \Delta A can be nonzero because the full definition is dA = -S dt - P dV + \mu dN, where N is the number of particles, and \mu is the chemical potential. So changing the number of particles can also change A. That's true, but doesn't really address the seeming contradiction, as far as I can see.
But here's the way that I think of the various kinds of "free energy". In the first place, imagine that you have two systems that are thermally isolated (no heat in or out) but share the same volume, as in figure 1:
Figure 1: Thermally isolated systems sharing a volume
If system S_1 expands by pushing the partition to the right, then it will do work on system S_2. The amount of work done is given by:
\Delta W = P \Delta V_1
We can use the definition of internal energy to relate the work to the heat going in or out:
\Delta U = \Delta Q - \Delta W = T \Delta S - P \Delta V
(Sometimes this is written with a plus sign in front of \Delta W[/tex], but that depends on whether you're talking about the work done by the system or the work done on the system. If the changes are reversible, as we're assuming, the two are just the negatives of each other).<br /> <br /> If the systems are thermally isolated, then \Delta Q = T \Delta S = 0, which means that \Delta W = - \Delta U<br /> <br /> So the work done by the system is just equal to the loss of internal energy of the system. So in this scenario, the internal energy is a measure of the ability of the system to do work. <br /> <br /> <b>Note</b>: The above discussion is for a reversible change. In an irreversible change, the work done by the system can be less than -\Delta U. So we really get:<br /> <br /> \Delta W \leq - \Delta U<br /> <br /> Now, let's relax the constraint that the two systems are thermally isolated, and instead assume that they are free to exchange heat with a surrounding medium, as in Figure 2:<br /> <div class="bbImageWrapper js-lbImage" title="Delta-A.jpg" data-src="https://www.physicsforums.com/attachments/delta-a-jpg.207957/" data-lb-sidebar-href="" data-lb-caption-extra-html="" data-single-image="1"> <img src="https://www.physicsforums.com/attachments/delta-a-jpg.207957/" data-url="" class="bbImage" data-zoom-target="1" style="" alt="Delta-A.jpg" title="Delta-A.jpg" width="800" height="503" loading="lazy" decoding="async" /> </div> <br /> <b>Figure 2: Systems may exchange heat with surrounding medium, but temperature is kept constant<br /> </b><br /> In this case, it's no longer necessarily true that \Delta Q = 0 when system 1 expands. As it expands, it can absorb heat from the surrounding heat bath, making \Delta Q &gt; 0. But assuming the heat bath is much larger than either of the two systems, the temperature will remain constant during any reversible change of system 1. So we can use a different thermodynamic identity:<br /> <br /> \Delta A = \Delta U - \Delta (S T) = - P \Delta V - S \Delta T<br /> <br /> In this case, the heat bath is keeping the temperature constant, so \Delta T = 0. So we have:<br /> <br /> \Delta A = -P \Delta V = -\Delta W<br /> <br /> So in this case, it is A that gives a measure of the potential of the system to do work, rather than U.<br /> <br /> Again, this is for a reversible change. For an irreversible change, -\Delta A is an upper bound on the amount of work done by the system:<br /> <br /> \Delta W \leq - \Delta A<br /> <br /> The discussion in Wikipedia is misleading, because of course, a system can't do any work if the volume is not allowed to change. I think that the issue is resolved by assuming that there is more than one system involved. The volume of the composite system doesn't change, but the volumes of the smaller subsystems can change, and Helmholtz free energy is a measure of the capacity of the subsystem to do work.
tze liu
- 57
- 0
is that your assumption?stevendaryl said:The discussion in Wikipedia is misleading, because of course, a system can't do any work if the volume is not allowed to change. I think that the issue is resolved by assuming that there is more than one system involved. The volume of the composite system doesn't change, but the volumes of the smaller subsystems can change, and Helmholtz free energy is a measure of the capacity of the subsystem to do work.
- 23,706
- 5,921
You are correct that no P-V work is done. So, if the only focus is P-V work, and the P-V work is zero, then the inequality is satisfied. But there are other kinds of work that can be done on the system (or the system can do on the surroundings), and, if these are operative, then the inequality applies. For a more detailed derivation and description of all this, see Denbigh, Principles of Chemical Equilibrium.tze liu said:Sorry,i want to ask a question here
the note said the volume is "fixed" here.
if the volume if fixed,how comes the work done(because no change of volume) here
i totally get lost here
thank
tze liu
- 57
- 0
""But there are other kinds of work that can be done on the system""Chestermiller said:You are correct that no P-V work is done. So, if the only focus is P-V work, and the P-V work is zero, then the inequality is satisfied. But there are other kinds of work that can be done on the system (or the system can do on the surroundings), and, if these are operative, then the inequality applies. For a more detailed derivation and description of all this, see Denbigh, Principles of Chemical Equilibrium.
thank
do u means those work done doesn't change the volume of the system?
- 23,706
- 5,921
Yestze liu said:""But there are other kinds of work that can be done on the system""
thank
do u means those work done doesn't change the volume of the system?
tze liu
- 57
- 0
when I study thermodynamic,Chestermiller said:Yes
i never heard that the work done doesn't change the volume of the system
So what is such "work done" looks like and how does this "work done" act on the system?
do u have any example?thank
- 23,706
- 5,921
Electrochemical work, stirring worktze liu said:when I study thermodynamic,
i never heard that the work done doesn't change the volume of the system
So what is such "work done" looks like and how does this "work done" act on the system?
do u have any example?thank
tze liu
- 57
- 0
why those work doesn't change the volume?Chestermiller said:Electrochemical work, stirring work
- 23,706
- 5,921
When you stir a liquid in a rigid closed container, does the volume of the container or the liquid change?tze liu said:why those work doesn't change the volume?
tze liu
- 57
- 0
oh i seeChestermiller said:When you stir a liquid in a rigid closed container, does the volume of the container or the liquid change?
the Work here is the free mechanical work.
but not contributed to the change of volume.
thank
- 23,706
- 5,921
Don't forget also the possibility of electrochemical work like a battery.tze liu said:oh i see
the Work here is the free mechanical work.
but not contributed to the change of volume.
thank
tze liu
- 57
- 0
Chestermiller said:Don't forget also the possibility of electrochemical work like a battery.
is this sentense incorrect?Chestermiller said:Don't forget also the possibility of electrochemical work like a battery.
even that the system is thermal isolated and the volume is fixed
there are STILL SOME electrochemical work or other type of mechanical work here
so the delta U is not zero?
Attachments
- 23,706
- 5,921
You can have a current-carrying wire coming into the container and another wire exiting the container, with the current driven by a battery situated inside the container (elecrochemical). In this case, the internal energy of the system in the container changes (i.e., the battery is running down). The current can drive a motor outside the container. So the system is doing work.tze liu said:is this sentense incorrect?
even that the system is thermal isolated and the volume is fixed
there are STILL SOME electrochemical work or other type of mechanical work here
so the delta U is not zero?
Lord Jestocost
tze liu said:Sorry,i want to ask a question here
the note said the volume is "fixed" here.
if the volume if fixed,how comes the work done(because no change of volume) here
i totally get lost here
thank
This is always confusing. The essential point is the following:
Calculate the free energy of a system at a given temperature T in state #1: F#1
Calculate the free energy of the system at a given temperature T in state #2: F#2
(to do such calculations you generally need statistical physics)
The work which is done when going from state #1 to #2 in course of a reversible, isothermal process (at temperature T ) is then
Wrev = F#2 - F#1
E.g., in case F#2 < F#1, ABS(F#2 - F#1) is the maximum work you can extract during this isothermal process.
EDIT: In case of irreversible processes you have (work done positive, work extracted negative sign):
ΔW ≥ ΔF
Last edited by a moderator:
tze liu
- 57
- 0
By the way,Is that true the reverior and the system both are at the same constant temperature in my case?
Chestermiller said:You can have a current-carrying wire coming into the container and another wire exiting the container, with the current driven by a battery situated inside the container (elecrochemical). In this case, the internal energy of the system in the container changes (i.e., the battery is running down). The current can drive a motor outside the container. So the system is doing work.
- 23,706
- 5,921
No. If you had checked out Denbigh like I suggested, you would have found that T is the temperature of the reservoir and also the temperature of the system at the interface with the reservoir. It is also the initial and final temperature of the system in the initial and final equilibrium states. But, during the process, it is not the temperature of the system (which is non-uniform) except at the interface with the reservoir.tze liu said:By the way,Is that true the reverior and the system both are at the same constant temperature in my case?
Chet
tze liu
- 57
- 0
however, there is dF=d(U-TS) in my equation.Chestermiller said:No. If you had checked out Denbigh like I suggested, you would have found that T is the temperature of the reservoir and also the temperature of the system at the interface with the reservoir. It is also the initial and final temperature of the system in the initial and final equilibrium states. But, during the process, it is not the temperature of the system (which is non-uniform) except at the interface with the reservoir.
Chet
how can they use the temperature in the reservoir but not using the temperature inside the system?
there are two T in this case
the T in the reservoir and the other T in my system which can be described by F free energy.
why the equation dF=d(U-TS) ignore the temperature change inside the system.
- 23,706
- 5,921
If the temperature is not uniform within your system during the process, then F (which is a function of equilibrium state) is undefined. If you want to define F within your system when it is not at equilibrium, then you have to integrate the local free energy per unit mass over the mass of your system.tze liu said:however, there is dF=d(U-TS) in my equation.
how can they use the temperature in the reservoir but not using the temperature inside the system?
there are two T in this case
the T in the reservoir and the other T in my system which can be described by F free energy.
why the equation dF=d(U-TS) ignore the temperature change inside the system.
Last edited:
tze liu
- 57
- 0
it seemsChestermiller said:No. If you had checked out Denbigh like I suggested, you would have found that T is the temperature of the reservoir and also the temperature of the system at the interface with the reservoir. It is also the initial and final temperature of the system in the initial and final equilibrium states. But, during the process, it is not the temperature of the system (which is non-uniform) except at the interface with the reservoir.
Chet
if the temperature is constant in my process, i assume this temperature is T2Chestermiller said:If the temperature is not uniform within your system during the process, then F (which is a function of equilibrium state) is undefined. If you want to define F within your system when it is not at equilibrium, then you have to integrate the local free energy per unit mass over the mass of your system.
and the environment temperature is T1 ( T1 cannot be T2,if so, then there is no heat transfer)
why it should be dF=d(U-T1S) but not dF=d(U-T2S) ?i don't know why they use the environment temperature in the equation but not using the system's temperature( assume the system has constant temperature)
- 23,706
- 5,921
As I said, see Denbigh.tze liu said:it seems
if the temperature is constant in my process, i assume this temperature is T2
and the environment temperature is T1 ( T1 cannot be T2,if so, then there is no heat transfer)
why it should be dF=d(U-T1S) but not dF=d(U-T2S) ?i don't know why they use the environment temperature in the equation but not using the system's temperature( assume the system has constant temperature)
- 23,706
- 5,921
Do you really think that, in an irreversible process, the temperature within the system is uniform?
From the Clausius inequality applied to this problem, we have: $$\Delta S=\frac{Q}{T}+\sigma$$ where Q is the heat transferred from the reservoir to the system, T is both the initial and final temperature of the system as well as the temperature of the reservoir (at the heat transfer boundary with the system), and ##\sigma## is the entropy generated within the system during the process (always positive). So we have: $$Q=T\Delta S-T\sigma$$
If we substitute this into the first law of thermodynamics, we obtain:
$$\Delta U=T\Delta S-T\sigma+W$$where W is the work done by the surroundings on the system. From this, it follows that $$W=\Delta F+T\sigma$$Since ##\sigma## is always positive, it follows that $$W\gt \Delta F$$
From the Clausius inequality applied to this problem, we have: $$\Delta S=\frac{Q}{T}+\sigma$$ where Q is the heat transferred from the reservoir to the system, T is both the initial and final temperature of the system as well as the temperature of the reservoir (at the heat transfer boundary with the system), and ##\sigma## is the entropy generated within the system during the process (always positive). So we have: $$Q=T\Delta S-T\sigma$$
If we substitute this into the first law of thermodynamics, we obtain:
$$\Delta U=T\Delta S-T\sigma+W$$where W is the work done by the surroundings on the system. From this, it follows that $$W=\Delta F+T\sigma$$Since ##\sigma## is always positive, it follows that $$W\gt \Delta F$$
Last edited:
jim mcnamara
Mentor
- 4,789
- 3,852
@tze liu - there is a free to download pdf version of what @Chestermiller referred to.
Hope you can get and read the book.
https://archive.org/details/ThePrinciplesOfChemicalEquilibrium
Chet - FWIW some folks in places outside the US have trouble finding or getting to sources via links - countrywide IP blacklistings, limits on search engines, etc.
Hope you can get and read the book.
https://archive.org/details/ThePrinciplesOfChemicalEquilibrium
Chet - FWIW some folks in places outside the US have trouble finding or getting to sources via links - countrywide IP blacklistings, limits on search engines, etc.
- 23,706
- 5,921
Here is the direct quote from Denbigh: "Consider the special case (a) that the only heat transferred to the system is from a heat reservoir which remains at the constant temperature T; (b) that the initial and final temperatures, ##T_1## and ##T_2##, of the system are equal, and are equal to the temperature T of the reservoir."
Note that this description fails to mention the important fact that the temperature of the system is not uniform during the process, or that it is equal to T only at the boundary with the reservoir (except in the initial and final states).
Note that this description fails to mention the important fact that the temperature of the system is not uniform during the process, or that it is equal to T only at the boundary with the reservoir (except in the initial and final states).
tze liu
- 57
- 0
Oh I see.than you very much .There are still problems here.if the temperature at the beginning are equal to the reverior,how comes the heat transfer between them at the beginning?(because it violates the zero law)
- 23,706
- 5,921
It is only at the boundary that they match. Normal to the boundary, the gas temperature is varying. And the average gas temperature is different from its temperature (and the reservoir temperature) at the boundary.tze liu said:Oh I see.than you very much .There are still problems here.if the temperature at the beginning are equal to the reverior,how comes the heat transfer between them at the beginning?(because it violates the zero law)
tze liu
- 57
- 0
Chestermiller said:Here is the direct quote from Denbigh: "Consider the special case (a) that the only heat transferred to the system is from a heat reservoir which remains at the constant temperature T; (b) that the initial and final temperatures, ##T_1## and ##T_2##, of the system are equal, and are equal to the temperature T of the reservoir."
Note that this description fails to mention the important fact that the temperature of the system is not uniform during the process, or that it is equal to T only at the boundary with the reservoir (except in the initial and final states).
that means the heat is transfer from the boundary to the gas,and cause the heat transfer from the heat bath to the system boundary?Chestermiller said:It is only at the boundary that they match. Normal to the boundary, the gas temperature is varying. And the average gas temperature is different from its temperature (and the reservoir temperature) at the boundary.
tze liu
- 57
- 0
tze liu said:Look very strange.are you sure the temperature are equ
that means the heat is transfer from the boundary to the gas,and cause the heat transfer from the heat bath to the system boundary?
thank youjim mcnamara said:@tze liu - there is a free to download pdf version of what @Chestermiller referred to.
Hope you can get and read the book.
https://archive.org/details/ThePrinciplesOfChemicalEquilibrium
Chet - FWIW some folks in places outside the US have trouble finding or getting to sources via links - countrywide IP blacklistings, limits on search engines, etc.
- 23,706
- 5,921
On the bath side of the boundary, the thermal conductivity of the heat bath liquid is taken to be very large, so that the magnitude of the temperature gradient in the bath liquid is very small, even for a finite heat flux. This is an inherent characteristic of an ideal heat bath. So there can be heat flow in the bath to- and from the boundary even though the temperature gradient is tiny.tze liu said:that means the heat is transfer from the boundary to the gas,and cause the heat transfer from the heat bath to the system boundary?
tze liu
- 57
- 0
oh i seeChestermiller said:On the bath side of the boundary, the thermal conductivity of the heat bath liquid is taken to be very large, so that the magnitude of the temperature gradient in the bath liquid is very small, even for a finite heat flux. This is an inherent characteristic of an ideal heat bath. So there can be heat flow in the bath to- and from the boundary even though the temperature gradient is tiny.
what causes the transfer of heat?
the different temperature between the gas and the system boundary created a heat flow?
- 23,706
- 5,921
If the gas is compressed, its temperature rises above that of the bath fluid, or if is expanded the opposite happens. Or if you electrically heat the gas, the temperature will rise above that of the bath fluid. Another example is a chemical reaction in the gas that is exotheic or endothermic. This all results in heat flow. So the heat flow is the effect, not the cause.tze liu said:oh i see
what causes the transfer of heat?
the different temperature between the gas and the system boundary created a heat flow?
tze liu
- 57
- 0
thank youChestermiller said:If the gas is compressed, its temperature rises above that of the bath fluid, or if is expanded the opposite happens. Or if you electrically heat the gas, the temperature will rise above that of the bath fluid. Another example is a chemical reaction in the gas that is exotheic or endothermic. This all results in heat flow. So the heat flow is the effect, not the cause.
i get confused in this part also
did they do something wrong?
for example Cv=(dQ/dT)v=T(dS/dT)v
however this is true only for reversible process
but the question doesn't state it is reversible
Attachments
- 23,706
- 5,921
The equation should really read $$C_v=\left(\frac{\partial U}{\partial T}\right)_v=T\left(\frac{\partial S}{\partial T}\right)_v$$ This a physical property relationship for the material that applies to any process. The problem with the example is the Q in the equation, which is path-dependent.tze liu said:thank you
i get confused in this part also
did they do something wrong?
for example Cv=(dQ/dT)v=T(dS/dT)v
however this is true only for reversible process
but the question doesn't state it is reversible
tze liu
- 57
- 0
do u think the Q in the textbook here is wrong also?Chestermiller said:The equation should really read $$C_v=\left(\frac{\partial U}{\partial T}\right)_v=T\left(\frac{\partial S}{\partial T}\right)_v$$ This a physical property relationship for the material that applies to any process. The problem with the example is the Q in the equation, which is path-dependent.
tze liu
- 57
- 0
but the definition of heat capacity is not dU/dt but dQ/dt~~Chestermiller said:The equation should really read $$C_v=\left(\frac{\partial U}{\partial T}\right)_v=T\left(\frac{\partial S}{\partial T}\right)_v$$ This a physical property relationship for the material that applies to any process. The problem with the example is the Q in the equation, which is path-dependent.
- 23,706
- 5,921
That's the freshman physics definition of heat capacity. When we got to thermodynamics, we learned that, if work is being done, the equation doesn't apply as well. The subscript v on Cv means that we can measure this physical property of the material by doing an experiment at constant volume and determining the amount of heat added. Then the measured Cv can be used with the other equations to do calculations even if the volume isn't constant.tze liu said:but the definition of heat capacity is not dU/dt but dQ/dt~~
tze liu
- 57
- 0
Chestermiller said:That's the freshman physics definition of heat capacity. When we got to thermodynamics, we learned that, if work is being done, the equation doesn't apply as well. The subscript v on Cv means that we can measure this physical property of the material by doing an experiment at constant volume and determining the amount of heat added. Then the measured Cv can be used with the other equations to do calculations even if the volume isn't constant.
the example in my textbook implies that dQ is reversible but forget to use dQrev?Chestermiller said:The equation should really read $$C_v=\left(\frac{\partial U}{\partial T}\right)_v=T\left(\frac{\partial S}{\partial T}\right)_v$$ This a physical property relationship for the material that applies to any process. The problem with the example is the Q in the equation, which is path-dependent.
- 23,706
- 5,921
Yes. But, now that you are using thermodynamics, it is no longer valid to use the definitions of the heat capacities in terms of Q. You should use them only in terms of U, H, and S. otherwise, you are going to get confused.tze liu said:the example in my textbook implies that dQ is reversible but forget to use dQrev?
tze liu
- 57
- 0
8
 did they make mistake again?
did they make mistake again?
tze liu
- 57
- 0
seems in this charter, they use the same logic where ds T=dQ again hereChestermiller said:Yes. But, now that you are using thermodynamics, it is no longer valid to use the definitions of the heat capacities in terms of Q. You should use them only in terms of U, H, and S. otherwise, you are going to get confused.
- 23,706
- 5,921
This is correct. Note the constant p.tze liu said:8View attachment 208098did they make mistake again?
tze liu
- 57
- 0
Chestermiller said:This is correct. Note the constant p.[/QUOT
constant pressure doesn't imply the heat transfer is reversible?
tze liu
- 57
- 0
constant pressure doesn't imply the heat transfer is reversible? that means Cp=(dQ/dT)p less or equal to T(dS /dT)pChestermiller said:This is correct. Note the constant p.
- 23,706
- 5,921
dQ=TdS only for a reversible path. A reversible path using dQrev is the only way we know of for calculating ##\Delta S## between two thermodynamic equilibrium states of a system.tze liu said:seems in this charter, they use the same logic where ds T=dQ again here
- 23,706
- 5,921
I don't know about that. I NEVER use differentials for an irreversible path.tze liu said:constant pressure doesn't imply the heat transfer is reversible? that means Cp=(dQ/dT)p less or equal to T(dS /dT)p
tze liu
- 57
- 0
how are u know it is correct.Chestermiller said:This is correct. Note the constant p.
because it seems they assume it is reversible here?
Cp=(dQrev/dT)p =T(dS /dT)p
however, if the dQ is not dQrev (for example: Cp=(dQ/dT)p)
this relationship seems to be wrong?
Lord Jestocost
The heat capacity at constant volume, CV, is the ratio δq/dT for a process in a closed constant-volume system with no nonexpansion work - that is, no work at all. The first law shows that under these conditions the internal energy change equals the heat: dU = δq. (Eq. 5.3.9). We can replace δq by dU and write CV as a partial derivative:
CV = (dU/dT )V
From: THERMODYNAMICS AND CHEMISTRY, SECOND EDITION, by Howard DeVoe, Associate Professor Emeritus, University of Maryland (http://www2.chem.umd.edu/thermobook/)
CV = (dU/dT )V
From: THERMODYNAMICS AND CHEMISTRY, SECOND EDITION, by Howard DeVoe, Associate Professor Emeritus, University of Maryland (http://www2.chem.umd.edu/thermobook/)
- 23,706
- 5,921
Please forget about using Q or Qrev. You need to switch to the following definitions to successfully use heat capacities in thermodynamics: $$C_v=\left(\frac{\partial U}{\partial T}\right)_v$$tze liu said:how are u know it is correct.
because it seems they assume it is reversible here?
Cp=(dQrev/dT)p =T(dS /dT)p
however, if the dQ is not dQrev (for example: Cp=(dQ/dT)p)
this relationship seems to be wrong?
$$C_p=\left(\frac{\partial H}{\partial T}\right)_p$$With these definitions, you can NEVER go wrong.
If you stick with Q, you are going to get confused when you consider processes that are neither at constant volume or constant pressure.
tze liu
- 57
- 0
Closed constant volume has no pv work but seems there may have other type of work done?Lord Jestocost said:The heat capacity at constant volume, CV, is the ratio δq/dT for a process in a closed constant-volume system with no nonexpansion work - that is, no work at all. The first law shows that under these conditions the internal energy change equals the heat: dU = δq. (Eq. 5.3.9). We can replace δq by dU and write CV as a partial derivative:
CV = (dU/dT )V
From: THERMODYNAMICS AND CHEMISTRY, SECOND EDITION, by Howard DeVoe, Associate Professor Emeritus, University of Maryland (http://www2.chem.umd.edu/thermobook/)
- 23,706
- 5,921
The total heat that flows is not required to be reversible. If the equation were written correctly, it would read:tze liu said:how are u know it is correct.
because it seems they assume it is reversible here?
Cp=(dQrev/dT)p =T(dS /dT)p
however, if the dQ is not dQrev (for example: Cp=(dQ/dT)p)
this relationship seems to be wrong?
$$C_p=\frac{Q}{(T_2-T_1)}=\frac{(S_2-S_1)}{\ln{(T_2/T_1)}}$$
where the subscripts 1 and 2 refer to the initial and final thermodynamic equilibrium states of the system, and where the pressure in state 1 is the same as the pressure in state 2, and the externally applied pressure to the system over the entire process path is the same as the in equilibrium states 1 and 2. Note that the heat does not have to be applied reversibly for this equation to be correct. During the process, the system (which is initially in thermodynamic equilibrium state 1), can be placed in contact with a reservoir at temperature ##T_2## and held in contact with this reservoir until the system reaches equilibrium in thermodynamic equilibrium state 2. The equation I wrote applies to this irreversible process equally well as to a process that took the system from state 1 to state 2 reversibly.
Similar threads
- Replies
- 55
- Views
- 3K
- Replies
- 24
- Views
- 2K
- Replies
- 24
- Views
- 3K
- Replies
- 8
- Views
- 2K
- Replies
- 3
- Views
- 2K
- Replies
- 8
- Views
- 2K
- Replies
- 34
- Views
- 4K
- Replies
- 2
- Views
- 2K
- Replies
- 20
- Views
- 2K
Hot Threads
-
I Explain Bernoulli at the molecular level?
- Started by user079622
- Replies: 251
- Classical Physics
-
B Simple mass/scale puzzle
- Started by DaveC426913
- Replies: 206
- Classical Physics
-
I Solving a momentum problem where masses change after the collision
- Started by rdemyan
- Replies: 35
- Classical Physics
-
I Topic about physics axioms, theory, laws etc..
- Started by user079622
- Replies: 93
- Classical Physics
-
I Two kinds of pressure
- Started by Demystifier
- Replies: 24
- Classical Physics
Recent Insights
-
Insights Why Entangled Photon-Polarization Qubits Violate Bell’s Inequality
- Started by Greg Bernhardt
- Replies: 26
- Quantum Interpretations and Foundations
-
Insights Quantum Entanglement is a Kinematic Fact, not a Dynamical Effect
- Started by Greg Bernhardt
- Replies: 11
- Quantum Physics
-
Insights What Exactly is Dirac’s Delta Function? - Insight
- Started by Greg Bernhardt
- Replies: 5
- General Math
-
Insights Relativator (Circular Slide-Rule): Simulated with Desmos - Insight
- Started by Greg Bernhardt
- Replies: 1
- Special and General Relativity
-
Insights Fixing Things Which Can Go Wrong With Complex Numbers
- Started by PAllen
- Replies: 7
- General Math
-
Insights Fermat's Last Theorem
- Started by fresh_42
- Replies: 105
- General Math

