Leoragon
- 43
- 0
A little background: I'm only a high school student with some knowledge on Lewis dot structures. And I don't know much about the s orbitals or p orbitals or whatnot.
Why are there lone pairs? Shouldn't the electrons repel each other? Why do we draw them as pairs?
For example: carbon dioxide is drawn like this
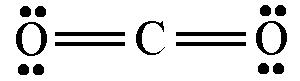
Why don't we draw it like
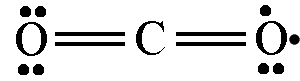
or
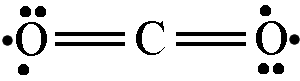
or sulfur dioxide
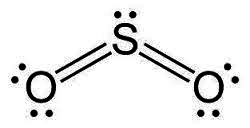
Why not this?
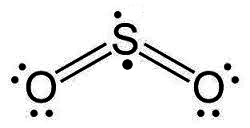
Are the drawings just arbitrary and the original used for simplicity's sake?
Can all these drawings work?
Why are there lone pairs? Shouldn't the electrons repel each other? Why do we draw them as pairs?
For example: carbon dioxide is drawn like this
Why don't we draw it like
or
or sulfur dioxide
Why not this?
Are the drawings just arbitrary and the original used for simplicity's sake?
Can all these drawings work?
Last edited: