You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Partition function Definition and 214 Threads
In physics, a partition function describes the statistical properties of a system in thermodynamic equilibrium. Partition functions are functions of the thermodynamic state variables, such as the temperature and volume. Most of the aggregate thermodynamic variables of the system, such as the total energy, free energy, entropy, and pressure, can be expressed in terms of the partition function or its derivatives. The partition function is dimensionless, it is a pure number.
Each partition function is constructed to represent a particular statistical ensemble (which, in turn, corresponds to a particular free energy). The most common statistical ensembles have named partition functions. The canonical partition function applies to a canonical ensemble, in which the system is allowed to exchange heat with the environment at fixed temperature, volume, and number of particles. The grand canonical partition function applies to a grand canonical ensemble, in which the system can exchange both heat and particles with the environment, at fixed temperature, volume, and chemical potential. Other types of partition functions can be defined for different circumstances; see partition function (mathematics) for generalizations. The partition function has many physical meanings, as discussed in Meaning and significance.
View More On Wikipedia.org
Each partition function is constructed to represent a particular statistical ensemble (which, in turn, corresponds to a particular free energy). The most common statistical ensembles have named partition functions. The canonical partition function applies to a canonical ensemble, in which the system is allowed to exchange heat with the environment at fixed temperature, volume, and number of particles. The grand canonical partition function applies to a grand canonical ensemble, in which the system can exchange both heat and particles with the environment, at fixed temperature, volume, and chemical potential. Other types of partition functions can be defined for different circumstances; see partition function (mathematics) for generalizations. The partition function has many physical meanings, as discussed in Meaning and significance.
View More On Wikipedia.org
-

How to turn partition sum into an integral?
In, *An Introduction to Thermal Physics, page 235*, Schroder wants to evaluate the partition function $$Z_{tot}=\sum_0^\infty (2j+1)e^{-j(j+1)\epsilon/kT}$$ in the limit that $kT\gg\epsilon$, thus he writes $$Z_{tot}\approx\int_0^\infty (2j+1)e^{-j(j+1)\epsilon/kT}\,dj$$ But how is this...- LightPhoton
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
E
Details regarding the high temperature limit of the partition function
My main question here is about how we actually justify, hopefully fairly rigorously, the steps leading towards converting the sum to an integral. My work is below: If we consider the canonical ensemble then, after tracing over the corresponding exponential we get: $$Z = \sum_{n=0}^\infty...- EE18
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-

I Average magnetic moment of atom in magnetic field ##B##
from the partition function - am trying to show that ##\langle \mu \rangle = \beta^{-1} (\partial \log Z / \partial B)## where ##Z## is the canonical partition function for one atom, i.e. ##Z = \sum_{m=-j}^{j} \mathrm{exp}(\mu_0 \beta B m)##, and ##\mu = \mu_0 m##. The average...- ergospherical
- Thread
- Replies: 1
- Forum: Quantum Physics
-
H
What Is the Correct Partition Function for a Spin System?
##Z = \sum_{-i}^{i} = e^{-E_n \beta}## ##Z = \sum_{0}^j e^{nh\beta} + \sum_{0}^j e^{-nh\beta}## Those sums are 2 finites geometric series ##Z = \frac{1- e^{h\beta(i+1)}}{1-e^{h\beta}} + \frac{1-e^{-h\beta(i+1)}}{1-e^{-h\beta}}## I don't think this is ring since from that I can't get 2 sinh...- happyparticle
- Thread
- Replies: 3
- Forum: Advanced Physics Homework Help
-
G
I How Is the Partition Function of BaTiO3 Calculated in a Cubic Lattice?
I have a cubic lattice, and I am trying to find the partition function and the expected value of the dipole moment. I represent the dipole moment as a unit vector pointing to one the 8 corners of the system. I know nothing about the average dipole moment , but I do know that the mean-field...- George444fg
- Thread
- Replies: 1
- Forum: Thermodynamics
-

Partition function of modified Ising model
$$H = - J ( \sum_{i = odd}) \sigma_i \sigma_{i+1} - \mu H ( \sum_{i} \sigma_i ) $$ So basically, my idea was to separate the particles in this way:: ##N_{\uparrow}## is the number of up spin particles ##N_{\downarrow}## "" down spin particles ##N_1## is the number of pairs of particles close...- LCSphysicist
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
H
I Partition function for continuous spectrum
Let's say that we have a one-particle Hamiltonian that admits only a continuous spectrum of eigenvalues ##E(k)=\alpha k^2## parameterized by asymptotic momentum ##\mathbf{k}## (assuming the eigenfunctions become planewaves far from the origin), would the partition function then be $$Z=\int...- HomogenousCow
- Thread
- Replies: 2
- Forum: Quantum Physics
-

I Derivation of the Canonical Ensemble
One of the common derivations of the canonical ensemble goes as follows: Assume there is a system of interest in the contact with heat reservoir which together form an isolated system. Heat can be exchanged between the system and reservoir until thermal equilibrium is established and both are...- Dario56
- Thread
- Replies: 3
- Forum: Thermodynamics
-

I Hamiltonian formalism and partition function
In hamiltonian formalism we have the generalized coordinates ##q_i## and the conjugates moments ##p_i##. For a dipole in a give magnetic field ##B## the Hamiltonian is ##H=-\mu B cos \theta## where ##\theta## is the angle between ##\vec \mu## and ##\vec B##. Can i consider ##\theta## or ##cos...- Simobartz
- Thread
- Replies: 1
- Forum: Classical Physics
-
S
I How Does Electron Spin Affect the Partition Function in Saha's Equation?
Hey, I have a question about proving Saha's equation for ionizing hydrogen atoms. The formula is \frac{P_{p}}{P_{H}} = \frac{k_{B} T}{P_{e}} \left(\frac{2\pi m_{e} k_{B}T}{h^2} \right)^{\frac{3}{2}}e^{\frac{-I}{k_{B} T}} with P_{p} pressure proton's, P_{H} pressure hydrogen atoms, m_{e}...- Sebas4
- Thread
- Replies: 1
- Forum: Thermodynamics
-
M
Partition Function for system with 3 energy levels
I determined the partition function of the particle A, B and C. C should be the same as B. I then considered the situation, where all particles are in the system at the same time, and drew a diagram of all possible arrangements: The grey boxes are the different partitions, given that we...- MigMRF
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
M
I Partition function of mixture of two gases
I have a question about statistical physics. Suppose we have a closed container with two compartments, each with volume V , in thermal contact with a heat bath at temperature T, and we discuss the problem from the perspective of a canonic ensemble. At a certain moment the separating wall is...- millerm
- Thread
- Replies: 1
- Forum: Other Physics Topics
-

How to find the partition function of the 1D Ising model?
Attempt at a solution: \begin{aligned}Z=\sum ^{N}_{r=0}C\left( N,r\right) e^{-\beta \left[ -NJ+2rJ\right] }\\ \Rightarrow Z=e^{\beta NJ}\sum ^{N}_{r=0}C\left( N,r\right) e^{-2\beta rJ}\end{aligned} Let ##e^{-2\beta J}=x##. Then ##e^{-2\beta rJ}=x^{r}##. \begin{aligned}\therefore Z=e^{\beta...- Dom Tesilbirth
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
I Phase space integral in noninteracting dipole system
Hi all, Consider a system of ##N## noninteracting, identical electric point dipoles (dipole moment ##\vec{\mu}##) subjected to an external field ##\vec{E}=E\hat{z}##. The Lagrangian for this system is...- raisins
- Thread
- Replies: 2
- Forum: Classical Physics
-
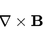
Grand partition function (Volume divided into N spaces)
equation i need to proof. the N in here, is the avarege number of particles, N0 is the total number of particles,V is total volume, v0 I am not quite sure what it is because it isn't mentioned in the homework, but I am assuming it is the volume of which space.- anaisabel
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-

Partition function of a particle with two harmonic oscillators
Here is the solution I have been given: But I really don't understand this solution. Why can I just add these two exponential factors (adding two individual partition...- mjmnr3
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
D
Rotational partition function for CO2 molecule
Hello fellow physicists, I need to calculate the rotational partition function for a CO2 molecule. I'm running into problems because I've found examples were they say this rotational partition function is: ##\zeta^r= \frac T {\sigma \theta_r} = \frac {2IkT} {\sigma \hbar^3}## Where...- DannyJ108
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-

I Help with an ideal gas canonical ensemble partition function integral
Where does the volume even come from? Any help would be appreciated!- AndreasC
- Thread
- Replies: 2
- Forum: Classical Physics
-

A Partition function in number theory and statistical mechanics
Is the partition function used in number theory and statistical mechanics the same thing?- qnach
- Thread
- Replies: 1
- Forum: General Math
-
S
Probability of a state given the partition function
If my partition function is for a continuous distribution of energy, can I simply say that the probability of my ensemble being in a state with energy ##cU## is ##e^{-\beta cU} /Z##? I believe that isn't right as my energy distribution is continuous, and I need to be integrating over small...- Sat D
- Thread
- Replies: 5
- Forum: Advanced Physics Homework Help
-

Classical Canonical Partition Function in Two Dimensions
For a single particle, $$Z=\frac{1}{h^2}\int_{-\infty}^{\infty} e^{-\beta \frac{P^2}{2m}}d^2p \int e^{-U(r)}drd\theta= \frac{1}{h^2}(\frac{2\pi m}{\beta}) 2\pi [\int_{0}^{r_0}e^{U_0}dr+\int_{r_0}^{R}dr]$$ $$ =\frac{1}{h^2}(\frac{2\pi m}{\beta}) 2\pi [e^{U_0}(r_0)+(R-r_0)]=\frac{\pi...- Diracobama2181
- Thread
- Replies: 4
- Forum: Advanced Physics Homework Help
-

Estimate the partition function by analyzing a graphic
I am not sure, but since the partition function Z is just the sum of all Boltzmann Factor We can just add: (some terms don't appear in the image, by the way, the estimative is nice, the result is above ANS) But i didn't understand what the author did: While i didn't even care about the...- LCSphysicist
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-
T
Exploring the Grand Partition Function for an Einstein Solid
$$Q_{(\alpha, \beta)} = \sum_{N=0}^{\infty} e^{\alpha N} Z_{N}(\alpha, \beta) \hspace{1cm} (3.127)$$ Where ##Q## is the grand partition function, ##Z_N## is the canonical partition function and: $$\beta = \frac{1}{kT} \hspace{1cm} \alpha = \frac{\mu}{kT} \hspace{1cm} (3.128)$$ In the case of an...- Ted Ali
- Thread
- Replies: 2
- Forum: Advanced Physics Homework Help
-

A Lennard Jones, 3 particles, partition function
Greetings, similar to my previous thread (https://www.physicsforums.com/threads/lennard-jones-potential-and-the-average-distance-between-two-particles.990055/#post-6355442), I am trying to calculate the average inter-particle distance of particles that interact via Lennard Jones potentials...- SchroedingersLion
- Thread
- Replies: 4
- Forum: High Energy, Nuclear, Particle Physics
-

I Partition function of quantum mechanics
In quantum mechanics, we have the partition function Z[j] = e-W[j] = ∫ eiS+ jiOi. The propagator between two points 1 and 2 can be calculated as ## \frac{\delta}{\delta j_1}\frac{\delta}{\delta j_2} Z = \langle O_1 O_2 \rangle## The S in the path integral has been replaced by S → S + jiOi...- PGaccount
- Thread
- Replies: 2
- Forum: Quantum Physics
-

Partition function from the density of states
I'm given the following density of states $$ \Omega(E) = \delta(E) + N\delta(E-\Delta) + \theta(E-\Delta)\left(\frac{1}{\Delta}\right)\left(\frac{E}{N\Delta}\right)^N $$ where $ \Delta $ is a positive constant. From here I have to "calculate the canonical partition function as a function of $$...- snatchingthepi
- Thread
- Replies: 3
- Forum: Advanced Physics Homework Help
-
P
Partition Function for Spin-1 One Dimensional Ising Model
$$H=-J\sum_{i=1}^{N-1}\sigma_i\sigma_{i+1}$$ There is no external magnetic field, so the Hamiltonian is different than normal, and the spins $\sigma_i$ can be -1, 0, or 1. The boundary conditions are non-periodic (the chain just ends with the Nth spin) $$Z=e^{-\beta H}$$...- pauladancer
- Thread
- Replies: 3
- Forum: Advanced Physics Homework Help
-
A
Partition function of 2 bosons in two energy level 0 and E
For 2 bosons each of which can occupy any of the energy levels 0 and E the microstates will be 3 0 E a a aa - - aa the partition function is therefore $$z=1+e^{-\beta E}+e^{-2\beta E}...(1)$$ Another approach to do.. The single particle partition function is $$z=1+e^{-\beta E} $$...- Apashanka
- Thread
- Replies: 9
- Forum: Advanced Physics Homework Help
-

A Getting structure data from a partition function?
Hello, From wikipedia, this is the partition function for a "classical continuous system": This is the pillar of classical statistical physics, but it can be seen as a mere kind of "mathematical transform" . It can be used even without thinking to statistics or temperature. If we focus only...- maajdl
- Thread
- Replies: 1
- Forum: Classical Physics
-
T
Solving Ising Spin 1 Model w/ Transfer Matrix Method
I did the first part using the transfer matrix method: $$ Z = Tr(T^{N}) $$ In this case, the transfer matrix will be $$ T(i,i') = \begin{pmatrix} e^{\beta J} & 1 & e^{-\beta J}\\ 1 &1 &1 \\ e^{-\beta J} & 1 & e^{\beta J} \end{pmatrix} $$ To get the trace of $T^N$, you find the...- Thales Castro
- Thread
- Replies: 6
- Forum: Advanced Physics Homework Help
-
W
I Grand Canonical Partition function
Hi everyone, I understand that the grand-canonical partition function is given by $$Z = \sum_i e^{-\beta(E_i - \mu N_i)}$$ Is there any interpretation to the quantity ##E_i - \mu N_i## here? In the canonical ensemble this would simply be energy of the ##i##th state, so I suppose this would be...- WWCY
- Thread
- Replies: 3
- Forum: Classical Physics
-
T
Stat-Mech problem: pressure from a partition function
Homework Statement A vessel having a volume ##V## initially contains ##N## atoms of dilute (ideal) helium gas in thermal equilibrium with the surroundings at a temperature ##T##, with initial pressure ##P_{i} (T ,V ) = \frac{NRT}{V}## . After some time, a number of helium atoms adhere to the...- TroyElliott
- Thread
- Replies: 4
- Forum: Advanced Physics Homework Help
-
L
A Partition function for a driven oscillator?
I've seen the partition function calculated for the SHO before in a thermodynamics course in order to calculate entropy. Is it possible to calculate it for a driven harmonic oscillator?- L_landau
- Thread
- Replies: 1
- Forum: Atomic and Condensed Matter
-
S
Canonical partition function of an ideal gas (unit analysis)
Homework Statement Basically the units of the Canonical Partition Function within the logarithms should be zero Homework Equations The Attempt at a Solution N here is a number so we ignore the left logarithms, applying a "Unit function " for the terms within the logarithm...- Somali_Physicist
- Thread
- Replies: 2
- Forum: Advanced Physics Homework Help
-
J
Where can I find rotational/vibrational temperature data for ethane?
Hi, Where would I find data for rotational/vibrational temperatures for a particular molecule (ethane)? I tried googling but had no luck. Also can you compute the moments of inerta for a particular (simple) molecule? -

I Derivation of the partition function
Starting from the definition of energy levels ##e_n## and occupations ##a_n## and the conditions ##\sum_n a_n = N## (2.2) and ##\sum_n a_n e_n = E## (2.3) where ##N## and ##E## are fixed I'm trying to find the distribution which extremizes the Shannon entropy. Using the frequency ##f_n=a_n/N##...- Mentz114
- Thread
- Replies: 5
- Forum: Quantum Physics
-
J
Entropy and the partition function for nitrogen
Homework Statement I'm attempting to calculate the translational entropy for N2 and I get a value of 207.8 J/Kmol. The tabulated value is given as 150.4 and I am stumped as to why the decrepancy. T = 298.15 K and P = 0.99 atm and V = 24.8 L R = 8.314 J/Kmol[/B] Homework Equations Strans =...- jbowers9
- Thread
- Replies: 6
- Forum: Introductory Physics Homework Help
-
J
Vibrational entropy and the partition function
Homework Statement I'm asked to compute the molar entropy of oxygen gas @ 298.15 K & 1 bar given: molecular mass of 5.312×10−26 kg, Θvib = 2256 K, Θrot = 2.07 K, σ = 2, and ge1 = 3. I'm currently stuck on the vibrational entropy calculation. Homework Equations [/B]S = NkT ∂/∂T {ln q} + Nk...- jbowers9
- Thread
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
H
I Help with a partition function calculation
<Re-opening approved by mentor.> Hi, I've always wondered why when calculating the partition function for a quantum system, we only sum over the eigenstates and not superimposed states. Thus I decided to actually try summing over all normalized states and see what would happen. Feedback is...- hammer123
- Thread
- Replies: 1
- Forum: Quantum Physics
-
H
I Help with partition function calculation
Hi, would very much appreciate it if I could get some help for something I was trying to calculate. I'm not very good at latex so I've just attached images of my attempt. I would very much appreciate it if you could look over the images I've taken and provide some feedback, thank you. I've been...- hammer123
- Thread
- Replies: 1
- Forum: Quantum Physics
-
D
Partition Function for N Quantum Oscillators
Homework Statement For 300 level Statistical Mechanics, we are asked to find the partition function for a Quantum Harmonic Oscillator with energy levels E(n) = hw(n+1/2). No big deal. We are then asked to find the partition function N such oscillators. Here I am confused. Homework Equations...- Daniel Sellers
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
R
Harmonic Oscillator and Volume of Unit Cell in Phase Space
Long time no see, PhysicsForums. Nevertheless, I have gotten myself into a statistical mechanics class where the prof is pretty brutal and while I can usually manage, this problem finally has me stumped. I'd like to be nudged in the right direction, not outright given the answer if possible. I... -
T
I Partition Function Derivation: Where Did I Go Wrong?
Self-repost from physics.SE; I underestimated how dead it was. So this follows Schroeder's Intro to Thermal Physics equations 6.1-6.7, but the question isn't book specific. Please let me be clear: I know for a fact I'm wrong. However, it feels like performing seemingly allowed manipulations, I...- thelaxiankey
- Thread
- Replies: 2
- Forum: Classical Physics
-

Partition Function of N particles in an assymetrical box
Homework Statement Consider a gas sufficiently diluted containing N identical molecules of mass m in a box of dimensions Lx, Ly, Lz. Calculate the probability of finding the molecules in any of their quantum states. Calculate the energy of each quantum state εr, as a function of the quantum... -
D
Deriving Fermi-Dirac Distribution misunderstanding
Homework Statement The actual question was deriving Bose-Einstein, but I got confused on the F-D example. I'm basically following the method given here. Homework Equations [All taken directly from the above link] Taylor series: The Attempt at a Solution So after that third equation... -

A Examples of fractal structure in prime partition numbers?
Regarding the recent discovery by Ken Ono and colleagues of the fractal structure of partition numbers for primes: a great lever of intuition would be to see a diagram, or any presentation of the numbers that reveals this fractal structure. Perhaps the fractal structure is somehow hidden in a...- Ventrella
- Thread
- Replies: 2
- Forum: General Math
-
A Zeros of the partition function (Yang-Lee and Fisher zeros)
Hey there, Just wondering where I can get a nice treatment of this with derivations. I could swear I read about this in Jon Cardy's Scaling and renormalization in statistical physics but I can't find it again so maybe I was wrong.- diegzumillo
- Thread
- Replies: 2
- Forum: Other Physics Topics
-

I Doubt about partition functions in QFT and in stat Mechanics
Hi, I was studying for my final exam on statistical physics and a doubt raised on my head that was truly strong and disturbing (at least, for me), and that I couldn't answer to myself by now. The doubt is: Given that we have in d dimensions a fermion non interacting gas, the statistical...- Iliody
- Thread
- Replies: 5
- Forum: Quantum Physics
-
C
Find magnetic susceptibility using partition function
Homework Statement A certain magnetic system contains n independent molecules per unit volume, each of which has four energy levels given by 0, ##Δ-gμ_B B##, ##\Delta##, ##\Delta +gμ_B B##. Write down the partition function, compute Helmholtz function and hence compute the magnetization ##M##...- cozycoz
- Thread
- Replies: 3
- Forum: Advanced Physics Homework Help
-

Partition Function at a Fixed Pressure
Homework Statement I don't quite follow the solution to this problem (problem 2.11 in Bergersen's and Plischke's textbook), here are the quoted problem and its solution: problem: solution: Homework EquationsThe Attempt at a Solution My problem is with the solution to (a), it seems they...- MathematicalPhysicist
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help