Thermodynamics Definition and 1000 Threads
-
R
Undergrad Does thermodynamics tell us that life will eventually end?
Hi. I apologize if this question has already been made. Unfortunately it's also difficult for me to formulate, especially in english. If you consider the Sun and Earth as an isolated system, where the Sun is able to produce energy indefinitely (it's not going to become a red giant), will life...- RaamGeneral
- Thread
- Life Thermodynamics
- Replies: 14
- Forum: Thermodynamics
-
D
Van der Waal expansion and delivered work
Homework Statement Assume that one mole of an ideal van der Waals fluid is expanded isothermally, at temperature T_h from an initial volume V_i to a final volume V_f. A thermal reselvoir at temperature T_c is available. Apply dW_{RWS} = \left ( 1 - \frac{T_{RHS}}{T} \right ) (-dQ) +(-dW) to a...- Dazed&Confused
- Thread
- Expansion Maximum work Second law of thermodyanmics Thermodynamics Work
- Replies: 1
- Forum: Introductory Physics Homework Help
-
C
Can Non-Spontaneous Reactions Occur When Coupled with Exergonic Reactions?
Greetings everyone! I have been a long-time lurker on here and have just recently signed up. I'm unsure if this was the correct forum to post this in, so I apologize in advance. My question isn't exactly homework per se - I am currently studying for my MCAT and have been trying to wrap my head...- ChasingZebras
- Thread
- Coupling Reaction Spontaneity Thermodynamics
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-

Isentropic Efficiency and Entropy Production Rate of Turbine
Homework Statement Water vapor at 6 MPa, 600C enters a turbine operating at steady state and expands to 10 kPa. The mass flow rate is 2 kg/s, and the power developed is 2626 kW. Stray heat transfer and kinetic and potential energy effects are negligible. Determine (a) the isentropic turbine...- AGiantGolden49er
- Thread
- Efficiency Entropy Isentropic Rate Steady state Thermodynamics Turbine
- Replies: 5
- Forum: Engineering and Comp Sci Homework Help
-

Thermodynamics Power Cycle Energy Balance
Homework Statement As shown in Fig. P5.39, a system undergoing a power cycle develops a net power output of 1 MW while receiving energy by heat transfer from steam condensing from saturated vapor to saturated liquid at a pressure of 100 kPa. Energy is discharged from the cycle by heat transfer...- AGiantGolden49er
- Thread
- Balance Control volume Cycle Energy Energy balance Power Thermodynamics
- Replies: 2
- Forum: Engineering and Comp Sci Homework Help
-

Thermodynamics Energy Balance: Solving for Work Input in an Isothermal Process
Question A mass of 1.5 kg of air at 120 kPa and 24 C is contained in a piston-cylinder device. The air is compressed to a final pressure of 600 kPa. During the process heat is transferred from the air such that the temperature inside the cylinder remains constant. Calculate the work input...- mastermechanic
- Thread
- Balance Energy Energy balance Thermodynamics
- Replies: 23
- Forum: Engineering and Comp Sci Homework Help
-
C
Undergrad Optimizing homemade ice cream freezing with brine
This question involves a bit of background, so please be patient. I understand that the heat absorbed by ice's phase change from solid to liquid is much greater than the amount that results from the difference in temperature of the ice and the ice cream batter. So the primary cooling effect is...- couldabin
- Thread
- Brine Freezing Homemade Ice Thermodynamics
- Replies: 8
- Forum: Thermodynamics
-

Classical Book for thermodynamics at undergrad level
I want to solve Irodov's problems in thermodynamics and for this I need a good textbook. I have Heat and Thermofynamics, Dittman and Zemansky. Should I read this book or should I go for another book?- Pushoam
- Thread
- Book Irodov Thermodynamics Undergrad
- Replies: 2
- Forum: Science and Math Textbooks
-

Explain to me the flaw in this perpetual motion scheme
Someone showed this to me, and I'm struggling to explain why this perpetual motion scheme is impossible. A picture: Basically, this is a cylinder within a large reservoir, with water at the level of the reservoir water level. The mass would be dropped and then reach the bottom of the cylinder...- ramzerimar
- Thread
- Explain Motion Perpetual motion Thermodynamics
- Replies: 2
- Forum: General Engineering
-

Thermodynamics Piston-Cylinder Question
Homework Statement Air contained in a piston–cylinder assembly, initially at 2 bar, 200 K, and a volume of 1 L, undergoes a process to a final state where the pressure is 8 bar and the volume is 2 L. During the process, the pressure–volume relationship is linear. Assuming the ideal gas model...- AGiantGolden49er
- Thread
- Thermodynamics
- Replies: 4
- Forum: Engineering and Comp Sci Homework Help
-

Thermodynamics: SFEE Homework Solution
Homework Statement This is a question from an exam that my university determined to be so harsh that they allowed resits with uncapped grades. I'm going to be taking such a resit thus I'm trying to figure out the paper: Air at 10oC and 80 kPa enters the diffuser of a jet engine steadily with...- JamesB93
- Thread
- Steady flow Thermodyamics Thermodynamics
- Replies: 3
- Forum: Engineering and Comp Sci Homework Help
-
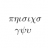
Graduate Fundamental thermodynamics relation
I was looking at the fundamental equation ##dU = Tds - Pdv + \sum_i \ \mu_i \ dN_i## and I was thinking of how many different ways one has for deriving it. I know I have to look through a book on Thermodynamics. I actually have done that some time ago and I will do that again. But the following...- davidge
- Thread
- Fundamental Relation Thermodynamics
- Replies: 3
- Forum: Thermodynamics
-
D
How Is Actual Power Calculated in a Turbine with Given Isentropic Efficiency?
Homework Statement Steam at a pressure of 4 MPa and 400 C and flow rate of 10 kg/s is expanded in a turbine to a pressure of 10 kPa. If the isentropic efficiency of the turbine is 0.85 then the actual power produced by the turbine is? Homework Equations :[/B] ηt= (m(h1-h2))/(m(h1-h2s)) = (W...- dzj633
- Thread
- Steam Thermodyamics Thermodynamics
- Replies: 2
- Forum: Engineering and Comp Sci Homework Help
-
J
Carnot cycle - Zero Power Extremes
Hey guys, I ran into this paper talking about the Maximum power you can obtain from a Carnot cycle: http://aapt.scitation.org/doi/abs/10.1119/1.10023 From what I understood, there are two extremes. To achieve maximum efficiency you have to make sure that the temperature of the system is never...- Joshua Pham
- Thread
- Carnot Carnot cycle Cycle Efficiency Power Thermodynamics Zero
- Replies: 7
- Forum: Mechanical Engineering
-
V
Thermodynamics, bullet melting ice problem
Homework Statement An ice cube at the melting temperature that has a mass of 20 g, is struck by a bullet with a mass of 9 g, flying at a certain speed. Determine the speed of the bullet, if it is known that one third of his energy was consumed to break the ice, and the remainder to melt it...- Vitalius6189
- Thread
- Bullet Ice Melting Thermodinamics Thermodynamics
- Replies: 4
- Forum: Introductory Physics Homework Help
-
T
Undergrad Thermodynamics: The Jet Engine
Good evening everyone,I had several questions regarding basic thermodynamics. After going to Paris Airshow (Le Bourget), lot's of questions came to my mind after looking at those beautiful Jet engines...I have always had a very basic understanding on these engines: a fan, a compressor (High and...- TheBusFlyer
- Thread
- Engine Jet Jet engine Thermodynamics
- Replies: 3
- Forum: Thermodynamics
-
A
Graduate Thermodynamics -- Entropy of an Isometric Process
In order to better explain my question let me give a precise situation and then state my question Say I have a well insulated rigid container containing some mass m of a saturated liquid-vapor mixture of water at some pressure P1. Initially it's at some quality x1. An electric resistance heater...- AnotherParadox
- Thread
- Entropy Process Thermodynamics
- Replies: 7
- Forum: Thermodynamics
-
V
Thermodynamics cycle work question
Homework Statement A mass of gas occupying volume V1 = 2 m3 at the pressure P1 = 4*10^5 Pa performs the cycle represented in the Figure that i have uploaded.What is the work of gas in this cycle, knowing that the pressure P2 = 10^5 Pa Homework Equations Work=1/2 * (P1 - P2) * (V1 - V3) The...- Vitalius6189
- Thread
- Cycle Physics Thermodynamics Work
- Replies: 7
- Forum: Introductory Physics Homework Help
-

Graduate Second law of thermodynamics and absolute zero
Does a system with zero entropy represent the thermal equilibrium at some temperature = 0K? Does the second law of thermodynamics entail that the system will eventually evolve to higher entropy? e.g. a system of 7 magnetic dipoles of paramagnetic spin-1/2 particles in an external magnetic...- i_hate_math
- Thread
- Absolute Absolute zero Law Second law Thermodynamics Zero
- Replies: 1
- Forum: Thermodynamics
-
A
Graduate Re-interpretation of the third law of thermodynamics
Consider the first paragraph of this paper - https://arxiv.org/abs/gr-qc/0611004: A fundamental problem in thermodynamic and statistical physics is to study the response of a system in thermal equilibrium to an outside perturbation. In particular, one is typically interested in calculating the...- Afonso Campos
- Thread
- Law Thermodynamics Third law
- Replies: 5
- Forum: Quantum Interpretations and Foundations
-

Thermodynamics: Steam is added to an ice cube
Homework Statement Vapors of 100 C is added to an ice cube of 0 C. How much of the ice cube has melted and what's the final temperature if the masses of the steam and the ice cube are 10.0 g & 50.0 g respectively? Homework Equations Lw = 3.33 x 105 J/kg cw = 4186 J/kg*C Q = m*c*ΔΤ Q =...- Const@ntine
- Thread
- Cube Ice Steady Steam Thermodynamics Vapor
- Replies: 16
- Forum: Introductory Physics Homework Help
-
J
Graduate Temperature change (first law of thermodynamics)
Hi, From the first law of thermodynamics it follows: Cp * (δT/δt) = (δQ/δt) where Cp = specific heat capacity, T = temperature, Q = heat, t = time From this formula, you would derive that temperature keeps on increasing as long as dQ/dt > 0. But if you, for example, look at the idealized...- jones123
- Thread
- Change Energy Energy balance Law Temperature Temperature change Thermodyamics Thermodynamics
- Replies: 4
- Forum: Thermodynamics
-

Internal Energy of an Ideal gas related to Molar specific heat
Homework Statement Please look at the below images which is the derivation of the relation between the internal energy of an ideal gas and the molar specific heat at constant volume. (Snaps taken from Fundamentals of Physics Textbook by David Halliday, Jearl Walker, and Robert Resnick) As...- SciencyBoi
- Thread
- Energy Gas Heat Ideal gas Internal Internal energy Kinetic theory of gases Specific Specific heat Thermodynamics
- Replies: 2
- Forum: Introductory Physics Homework Help
-
F
Graduate Thermodynamics - isentropic, polytropic and compressibility
Several questions: What does compression actually means? In the case of isentropic calculations a changed in density (or specific volume) is included in the calculations (isentropic.jpg) so isentropic means that compressibility is included in those calculations or what?In a previous thread, a...- Friis
- Thread
- Compressibility Isentropic Polytropic Thermodynamics
- Replies: 1
- Forum: Thermodynamics
-
A
How Does Adiabatic Compression Affect Air Temperature and Volume?
. Consider a pump that is required to compress air in a factory. The cylinder in the pump has an inner diameter of 2.00 cm and length 60.0 cm. Air is drawn into the pump at atmospheric pressure and 18 degrees celsius and the pump adiabatically compresses the air to a pressure of 17...- Akaramos45
- Thread
- adiabatic compression thermodynamics
- Replies: 1
- Forum: New Member Introductions
-

Courses Should I take a physics course over summer or is it cramming
At my community college, the first calculus based physics courses (the predecessor to modern physics) are split into 3 semesters: mechanics, electromagnetism, and thermodyamics/light. As long as you take the mechanics part first, you can choose which one to take next. This summer session is 8...- T dawg
- Thread
- course physics school summer thermodynamics
- Replies: 8
- Forum: STEM Academic Advising
-

Thermodynamic assembly - Statistical Thermodynamics
Homework Statement Consider a model thermodynamic assembly in which the allowed (nondegenerate) states have energies 0, ε, 2ε, 3ε.The assembly has four distinguishable (localized) particles and a total energy U = 6ε. Tabulate the nine possible distributions of the four particles among the...- PePaPu
- Thread
- Assembly Distribution Statistical Statistical thermodynamics Thermodynamic Thermodynamics
- Replies: 1
- Forum: Introductory Physics Homework Help
-
M
Computation of maximum pressure in heated closed vessel
I have a relatively simple design problem but my memories of thermodynamics are very rusty and I can't figure it out on my own. To make it short, I want to put a mix of solid, water and air in a 500mL pressure vessel and heat it all up to 250'C for several weeks. T and P are at room conditions...- Mattoo
- Thread
- Closed Closed system Computation Heating Maximum Pressure Pressure vessel Thermodynamics Vessel
- Replies: 3
- Forum: Mechanical Engineering
-
B
High School What is the comparison between the Born rule and thermodynamics?
Some say there is analogy in the Born rule in thermodynamics where the particles locations depend on the probabilities. In QM, the amplitude square is where you have the probability of the particles being there. How about in thermodynamics.. what is the counterpart of the Born rules, and what...- Blue Scallop
- Thread
- Born rule Thermodynamics
- Replies: 95
- Forum: Quantum Physics
-

Undergrad Does Building Construction Defy the Second Law of Thermodynamics?
From what I know, the law says that disorder increases over time. But, when a building is constructed the disordered bricks,cement etc. take form of the ordered building. Am I wrong or is this an exception?- shihab-kol
- Thread
- Disorder Law Second law Second law of thermodyanmics Thermodynamcics Thermodynamics
- Replies: 10
- Forum: Thermodynamics
-

Graduate Proof of fundamental thermodynamics equation for open systems
Hi ! I'm having a bit of trouble understanding something. Let 'u' be internal energy, 'h' enthalpy, 'e' work and 'q' heat. ('r' are dissipations and 'S' entropy) From a book , i read that de+dr=PdV= -du + TdS This seems to stand for closed cycle. Yet, my teacher uses the formula de+dr=vdP=...- PHstud
- Thread
- Fundamental Proof Systems Thermodynamics
- Replies: 3
- Forum: Thermodynamics
-

How Does Altitude Affect Humidity Sensor Readings?
Homework Statement So I sent a humidity sensor up in a weather balloon, which gave a reading for relative humidity. I eventually want an absolute humidity, but I am unsure whether I need to correct the output based on the changing density and atmospheric pressure with altitude. Homework...- oobgular
- Thread
- Altitude Change Humidity Physics Thermodynamics Vapor Water
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
G
The relation between the energy minimum and entropy maximum
Hi. This is the problem 5.1-1 from the second edition of Callen's Thermodynamics. It says Formulate a proof that the energy minimum principle implies the entropy maximum principle. That is, show that if the entropy were not maximum at constant energy then the energy could not be minimum at...- Gabriel Maia
- Thread
- Energy Entropy Maximum Minimum Relation Thermodynamics
- Replies: 1
- Forum: Advanced Physics Homework Help
-

Chemistry: First principle of thermodynamics
Homework Statement https://holland.pk/uptow/i4/1ca04ddf7c25f6c63e307720c34a4ff3.png Homework Equations First principle of thermodynamics: E=q+w The Attempt at a Solution E=q+w=-17+21=4kJ>0 ∴Endothermic (absorb heat from surrounding) (a) false (from surrounding to the system) (b) false (does...- yecko
- Thread
- Chemistry Principle Thermodynamics
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
B
Graduate Maximum work done in a Carnot Cycle
Given that in a Carnot Cycle the two adiabatic processes are essentially equal and opposite in magnitude the total work done by the cycle is in the two isotherms. The total work of the system is generally given as -NR(Th-Tc)ln(Vb/Va). Does this mean that the work done by a monatomic ideal gas is...- BrianSauce
- Thread
- Carnot Carnot cycle Cycle Maximum Maximum work Thermodynamics Work Work done
- Replies: 6
- Forum: Thermodynamics
-
M
Graduate Naive Doubt About Thermodynamic Equilibrium
This is more of a recurring conceptual doubt that I keep on running into when solving thermodynamics problems. We are taught that variations between extensive state variables in equilibrium are given by the following 'fundamental formula': dE = TdS + \mathbf{J}\cdot{d}\mathbf{x} +...- modulus
- Thread
- Doubt Equilibrium Internal energy Thermodynamic Thermodynamics
- Replies: 10
- Forum: Thermodynamics
-
C
Calculate the moisture content of air
Homework Statement (Simplified) During tests at cliffside power station, the following data was recorded from the cooling tower. A cooling tower has a 9 cell draft design, each diameter is 10m. Data D_o = 10m V_exit = 14.4 m/s T_exit(15 celsius) = 288.15K Ambient conditions : T(15 celsius) =...- clurt
- Thread
- Air Evaporation Humidity Temperature Thermodynamics
- Replies: 2
- Forum: Engineering and Comp Sci Homework Help
-
L
Graduate Thermodynamics. Partial derivative tricks.
If we consider function ##z=z(x,y)## then ##dz=(\frac{\partial z}{\partial x})_ydx+(\frac{\partial z}{\partial y})_xdy##. If ##z=const## then ##dz=0##. So, (\frac{\partial z}{\partial x})_ydx+(\frac{\partial z}{\partial y})_xdy=0 and from that \frac{dx}{dy}=-\frac{(\frac{\partial z}{\partial...- LagrangeEuler
- Thread
- Derivative Partial Partial derivative Thermodynamics
- Replies: 4
- Forum: Thermodynamics
-

Heat transfer in series and parallel
Homework Statement Homework Equations P = k A \frac{dT}{dx} The Attempt at a Solution a) Assuming steady state transfer, energy transfers through rods at the same rate everywhere. Letting T be the temperature at the point of welding. P_1 = k_1 A \frac{T_h-T}{L} \\ P_2 = k_2 A \frac{T -...- knc
- Thread
- Heat Heat transfer Parallel Physics Series Thermal conductivity Thermodynamics
- Replies: 11
- Forum: Introductory Physics Homework Help
-
L
Undergrad Why Do Authors Use γ=γ(P,T) Instead of γ=γ(P,T,V) in Thermodynamics?
Thermal coefficient of pressure is defined by \gamma=\frac{1}{P}(\frac{\partial P}{\partial T})_V . Why in books authors uses ##\gamma=\gamma(P,T)## and no ##\gamma=\gamma(P,T,V)##. Could you explain me this. I am sometimes confused with this dependences in thermodynamics.- LagrangeEuler
- Thread
- Relations Thermodynamics
- Replies: 4
- Forum: Thermodynamics
-

Calculating work of Otto cycle stages - Thermodynamics.
One big coincidence of thermodynamics is that automobiles are usually powered by an Otto cycle This cycle consists of an adiabatic compression (the cylinder compresses), isochoric compression (the fuel ignites, increasing the temperature in too short a time for the piston to move), adiabatic...- Pull and Twist
- Thread
- Cycle Engine Engineering Otto Physic Thermodyamics Thermodynamics Work
- Replies: 1
- Forum: Introductory Physics Homework Help
-
W
Undergrad Thermodynamic cycle of a solar cell
Mechanical Engineering Undergrad here: So this didn't seem explicitly like a homework question, but I was wondering how to describe a solar cell in terms of thermodynamics. If I had to model it with the first law of thermodynamics, I would consider it a simple loop of heat/work exchange...- whitejac
- Thread
- Cell Cycle Energy Solar Solar cell Thermodynamic Thermodynamics
- Replies: 1
- Forum: Thermodynamics
-

Steam generation thermodynamics
a cylindrical tank with volume V a volume of water is inside the tank and the rest is air now if coal combustion is releasing heat into the tank Qin and when the gauge reads a certain pressure "P", the valve is opened which allows steam to exit the boiler what's the mass flow rate of the steam?- Ali Ahmad
- Thread
- Generation Steam Thermodynamics
- Replies: 12
- Forum: Mechanical Engineering
-
S
Assumption of local thermodynamic equilibrium in a fluid
Moving fluids are generally in a state of non-equilibrium. However, in fluid dynamics, people generally assume a state of local thermodynamic equilibrium and argue that in such a condition, equilibrium thermodynamic concepts such as pressure, temperature, entropy, internal energy etc. can be...- Shivam Sinha
- Thread
- Equilibrium Fluid Fluid dynamics Fluids Local Non-equilibrium Thermodynamic Thermodynamics
- Replies: 10
- Forum: Mechanical Engineering
-
A
Graduate Conservation of entropy -- adiabatic process
Hello.I have a question about entropy of a thermodynamic system. 1)If we have let say a gas that is separated by some thermo isolated walls (so no heat goes in or out) does the entropy of that gas conserve? I taught that if S=dQ/dt, because Q=0,then the entropy should be conserved. 2)So,does the...- AlexS
- Thread
- Adiabatic Adiabatic process Conservation Entropy Process Thermodynamics
- Replies: 1
- Forum: Thermodynamics
-
S
Understanding Internal Energy Changes in Phases of Water
Homework Statement Sketch a diagram of internal energy (y-axis) versus temperature in the range from -10°C to +112°C to indicate how energy would change for a fixed quantity of water in its three phrases. Label and explain the main features of the variation. Homework Equations The Attempt at...- SpiraRoam
- Thread
- Energy Internal Internal energy Thermodynamics
- Replies: 8
- Forum: Introductory Physics Homework Help
-
M
Gas Compression in a Closed System: Non-Linear vs. Horizontal P-V Diagrams
When a gas in a closed system is compressed, should the graph always be non-linear? If T is constant ie if the process isothermal it is clear that it should be so but I am not very sure that if the graph can be horizontal. If T decreases might not it be horizontal? Thank you.- mech-eng
- Thread
- Diagram P-v diagram Thermodynamics
- Replies: 4
- Forum: Mechanical Engineering
-
S
Undergrad Confusion regarding the First Law of Thermodynamics
Is forcing a closed system to expand (e.g. by pulling out a piston), causing it to cool, work done to the system or work done by the system? I assume it was work done to the system, but that means the first law of thermodynamics formula no longer balances if you assume an adiabatic change...- Smith
- Thread
- Confusion First law Law Thermodynamics
- Replies: 4
- Forum: Thermodynamics
-
T
How Long Should My Double Pipe Heat Exchanger Be for a 22kW Capacity?
I'm trying to design a double pipe heat exchanger for two different water lines. One line is chilled water of approximately 7degC and the other is chilled water return from an air handling unit, of around 12degC. I want to design this heat exchange for a capacity of approximately 22kW. The part...- thestudent101
- Thread
- Heat Heat exchanger Heat transfer Pipe Thermodynamics
- Replies: 2
- Forum: Mechanical Engineering
-
S
Thermodynamics Question Regarding assuming Irreversibilities
Homework Statement There exists a tank filled with air with a given volume, temperature, and pressure. The tank exists in a room at a given temperature and pressure. That is: For the tank: P=1MPa, T=700k, V=1m^3 Outside: T=295K, P=100kPa Homework Equations \psi 2-\psi...- ScareCrow271828
- Thread
- Entropy Thermodynamics
- Replies: 9
- Forum: Engineering and Comp Sci Homework Help