SUMMARY
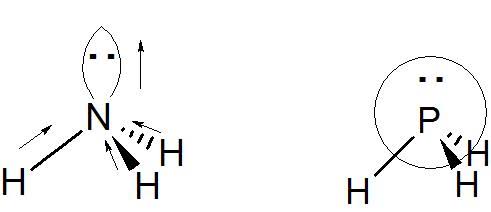
The discussion centers on the concept of the orbital dipole moment associated with lone pairs in chemical bonding, specifically in the context of nitrogen and hydrogen interactions. Participants clarify that the dipole moment for lone pairs points from the central atom (Nitrogen) towards the lone pair orbital, which is consistent with the electronegativity of the atom. The conversation highlights the difference between chemistry and physics conventions regarding dipole moment directionality, emphasizing that in chemistry, dipole moments are represented from δ+ to δ-, while in physics, they point towards positive charges. The discussion also references a specific educational resource for further clarification on this topic.
PREREQUISITES
- Understanding of chemical bonding principles
- Familiarity with dipole moments and electronegativity
- Knowledge of hybridization in molecular geometry
- Awareness of conventions in chemistry versus physics regarding dipole representation
NEXT STEPS
- Research the concept of hybridization in molecular geometry
- Study the differences between dipole moments in chemistry and physics
- Explore the role of lone pairs in molecular polarity
- Review educational resources on chemical bonding, such as the one mentioned in the discussion
USEFUL FOR
Chemistry students, educators, and professionals seeking to deepen their understanding of molecular dipole moments and the implications of lone pairs in chemical bonding.