- #1
gianeshwar
- 226
- 14
Summary:: How internal energy changes in a acclerated cabin.
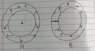
There are two tourus shaped insulated closed pipes containing equal amounts of ideal gas under same conditions.
B has a adiabatic partion wall.
If both are to change angular velocity by w radians per second.
How internal energies will change in both?
There are two tourus shaped insulated closed pipes containing equal amounts of ideal gas under same conditions.
B has a adiabatic partion wall.
If both are to change angular velocity by w radians per second.
How internal energies will change in both?