- #1
Pushoam
- 962
- 51
Homework Statement
Homework Equations
The Attempt at a Solution
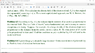
##\rho = b r
\\E =\frac { k r^2} {4ε_0}
\\ p ∝ E^a
\\E \left ( r=d \right ) = \frac { k d^2} {4ε_0}
\\ p = q d
\\ d ∝ d^{2a}
\\a = ½
\\p ∝ √E ##
PART B
##\rho ∝ r^n
\\E ∝r^{n+1}
\\ p ∝ E^a
\\d ∝d ^{{n+1}a}
\\## For eq. 4.1 to hold, ## a = \frac 1 { n+1} =1
\\ n = 0##
So, rho should be constant.